-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(11): 273-277
doi:10.5923/j.ijge.20251311.03
Received: Oct. 7, 2025; Accepted: Nov. 3, 2025; Published: Nov. 14, 2025

A Review of the Genetic Diversity of Monilinia Laxa (Aderh. & Ruhland) Honey and Its Distribution in Uzbekistan
Juraqulov Jahongir Juraqul ugli1, Mustafayev Ilyor Muradullayevich2, Samatova Shohista Azamatovna3
1PhD Student, Institute of Botany of the Academy of Sciences, Tashkent, Uzbekistan
2PhD of Biological, Senior Researcher, Tashkent Botanical Garden Named after F.N. Rusanov at the Institute of Botany of the Academy of Sciences, Tashkent, Uzbekistan
3PhD of Biological, Professor, Karshi State University, Tashkent, Uzbekistan
Correspondence to: Juraqulov Jahongir Juraqul ugli, PhD Student, Institute of Botany of the Academy of Sciences, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Monilinia laxa (Aderhold & Ruhland) Honey, the causal agent of brown rot blossom and twig blight, is one of the most destructive fungal pathogens affecting stone fruit crops worldwide. The fungus primarily infects blossoms, spurs, twigs, and fruits, leading to significant yield and quality losses, particularly under humid and moderately warm conditions. This review summarizes global research on the genetic diversity of M. laxa and presents new data on its distribution and incidence in fruit orchards across Uzbekistan. Field surveys and mycological analyses were carried out in major fruit-growing regions, including Tashkent, Namangan, Samarkand, Bukhara, and Khorezm, to assess disease severity and cultivar susceptibility. The results revealed that M. laxa is widely distributed across Uzbekistan, with the highest infection rates (up to 55%) observed in regions characterized by moderate humidity and rainfall. Apricot cultivars such as “Shalax”, “Qora orik”, and “Gulungi luchchak”, and cherry cultivars “Valeriy Chkalov” and “Savri surkhoni” were highly susceptible, whereas “Isfarak”, “Ruhi juvanon”, and “Nikitin” demonstrated relative resistance. Global studies employing molecular markers such as RAPD, AFLP, ISSR, and RFLP have revealed high intraspecific genetic diversity within M. laxa populations, often independent of host species or geographical origin. This genetic variability, combined with environmental influences, contributes to the pathogen’s adaptability and persistence in orchard ecosystems. The findings emphasize the importance of integrated disease management strategies in Uzbekistan, including the use of resistant cultivars, proper sanitation, and targeted fungicide application, supported by continuous monitoring of pathogen population dynamics.
Keywords: Pathogenic fungi, Brown rot, Genetic diversity, Stone fruits, Disease incidence, Disease intensity, Cultivar resistance, Disease distribution
Cite this paper: Juraqulov Jahongir Juraqul ugli, Mustafayev Ilyor Muradullayevich, Samatova Shohista Azamatovna, A Review of the Genetic Diversity of Monilinia Laxa (Aderh. & Ruhland) Honey and Its Distribution in Uzbekistan, International Journal of Genetic Engineering, Vol. 13 No. 11, 2025, pp. 273-277. doi: 10.5923/j.ijge.20251311.03.
1. Introduction
- Pathogenic fungi belonging to the genus Monilinia Honey are currently recognized as one of the major limiting factors in fruit production worldwide. These fungi primarily affect fruit-bearing trees and shrubs of the Rosaceae family, causing varying degrees of yield loss and significantly reducing both the quantity and quality of the harvest. Monilinia infections manifest in plants through a range of symptoms, including blossom and twig blight, fruit rot, and leaf damage. In recent years, the geographic distribution of this disease has expanded, making it economically significant not only in European countries but also in Asia, North America, and other regions. For example, in Serbia in 1999, up to 100% of cherry trees were affected during the flowering period and on twigs [1]. In California, fruit losses during storage exceeded 30%, while in Spain, storage-related losses reached up to 59% in certain years. Even as early as the 1950s, complete yield losses of nectarine crops were reported in some areas [2]. These data indicate that Monilinia infections can cause severe economic damage not only in the field but also during postharvest storage. Brown rot blossom and twig blight, incited by Monilinia laxa (Aderhold & Ruhland) Honey, represents one of the most destructive diseases affecting stone fruit species. The pathogen is considered endemic in Europe and is widespread across major fruit-producing regions characterized by humid and moderately warm climates M. laxa is responsible for blossom, spur, and twig blight, as well as brown rot symptoms on fruits. The sexual (teleomorphic) stage of this fungus is rarely observed. Consequently, the pathogen typically overwinters in infected plant tissues such as twig cankers, blighted blossoms, peduncles, and mummified fruits. During spring, the mycelium within these overwintering structures resumes growth and produces abundant conidia, which serve as the primary inoculum source. When conidia are dispersed and deposited on susceptible tissues including blossoms, spurs, and young twigs – new infections are initiated. As the growing season progresses, especially during the fruit maturation stage, fruits become increasingly susceptible to M. laxa infection. Under favorable environmental conditions, these infections may escalate rapidly, leading to severe epidemics by harvest time. The infected fruits eventually become mummified, remaining attached to trees or on the ground, and act as an important source of inoculum for the subsequent growing season [3,4].The main objective of this study is to review the research conducted worldwide on the genetic diversity of M. laxa and to assess its distribution in the fruit orchards in Uzbekistan.
2. Materials and Methods
- Study object and sample collectionThis study was conducted as a bibliographic review incorporating elements of comparative analysis on the genetic diversity of M. laxa populations. Relevant literature was systematically retrieved from international scientific databases, including Scopus, Web of Science, PubMed, Google Scholar, and ScienceDirect, focusing on publications released after 2000. For the experimental component, plant samples infected with Monilinia spp. were collected from fruit orchards located in the Tashkent, Bukhara, Namangan, Surxondaryo, Navoiy, Jizzakh, Sirdaryo, and Khorezm regions of Uzbekistan (Fig. 1).
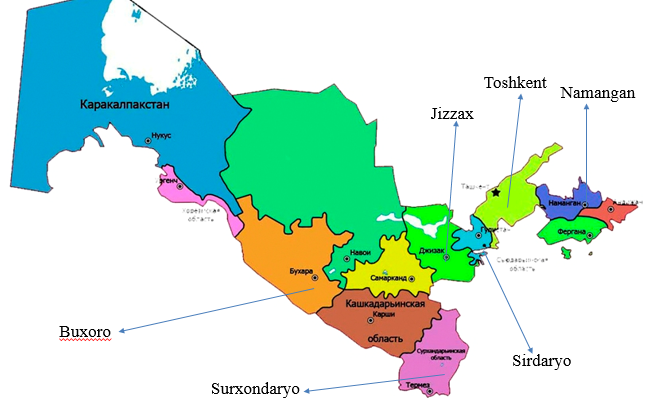 | Figure 1. Study areas across Uzbekistan |
3. Results and Discussion
- The assessment of genetic diversity within and among populations of fungal species has been a central topic in plant pathology research. Various molecular techniques, including inter-simple sequence repeat (ISSR), restriction fragment length polymorphism (RFLP), amplified fragment length polymorphism (AFLP), and randomly amplified polymorphic DNA (RAPD) fingerprinting, have been widely applied to analyze the genetic variability of fungal pathogens [7,8,10,11,12]. Within the Monilinia genus, studies on genetic diversity have primarily focused on M. fructicola [9,10,12], whereas similar investigations on M. laxa have been comparatively limited [7,8,9]. These studies collectively highlight the usefulness of molecular fingerprinting techniques for understanding population structure, evolutionary relationships, and the epidemiology of Monilinia species.In studies examining the genetic diversity of Monilinia laxa, Fulton et al. reported population-level variation that appeared to be randomly distributed on a global scale based on RAPD marker analyses [4]. In one of the first extensive investigations of M. laxa populations, Gell et al. (2007) demonstrated that the grouping of M. laxa isolates was independent of their geographical origin, indicating no clear pattern related to whether isolates originated from the same or different orchards [7]. Furthermore, the authors found no relationship between the clustering of isolates and the year of collection or host species.In contrast, Gril et al. (2008) identified significant genetic differentiation between isolates obtained from apple trees and those collected from other host plants. However, no additional grouping related to other host species was detected. Their study, which utilized an AFLP marker system, provided further insight into the genetic diversity and host-related variation within M. laxa populations [8].During rainy spring seasons, M. laxa can lead to significant crop losses in stone fruit orchards [3]. The severity of blossom blight can often be managed in conventionally grown orchards through one to three applications of protective or systemic fungicides during the flowering period, depending on local weather conditions [13]. However, under favorable environmental conditions, controlling brown rot remains challenging [2,3]. One of the main difficulties in disease management stems from the limited understanding of the genetic structure of M. laxa populations [14,7]. To date, only a few studies have explored the relationship between fungicide treatments and the genetic composition of plant pathogen populations [4]. Fazekas et al. (Hungary) showed that the clustering of M. laxa isolates was independent of host species, corroborating earlier observations by Gell et al., who reported no grouping according to peach, almond, or apricot [4]. In contrast, Gril et al. (2008) found host-specific differentiation between apple and other hosts, although no further clustering among stone fruits was detected, consistent with the Hungarian results [8]. The study also demonstrated that most fungicides effectively inhibited M. laxa mycelial growth regardless of geographic origin or host, with some isolates exhibiting reduced sensitivity to boscalid – piraclostrobin, elementary sulphur, cyprodinil, fenhexamid, and prochloraz. Reduced efficacy was generally associated with frequent application in seasonal programs, except for elementary sulphur, whose inherently low activity against Monilinia spp. is well documented. In conclusion, the Hungarian M. laxa population displays high genetic diversity independent of location, host, inoculum source, or fungicide sensitivity. This variation likely arises from pathogen migration among orchards and hosts, underscoring the key role of sanitation practices in the management of brown rot at both local and regional scales [4].As a result of mycological investigations conducted across various regions of Uzbekistan, samples of fruit trees infected with by M. laxa were collected. Based on these samples, the distribution characteristics of the disease, the degree of plant infection, and specific symptomatic features were analyzed. For each crop species and cultivar, the incidence of M. laxa, morphological manifestations of the disease, and infection severity were evaluated. The results indicated that the pathogen is widely distributed in certain fruit tree species, while in some cases, severe damage to fruits and vegetative parts was observed. Detailed observations on disease incidence and symptomatic features for each crop species are presented below.Apricot (Prunus armeniaca L.)Apricot brown rot caused by M. laxa is a highly destructive fungal disease that significantly reduces yield and fruit quality. The pathogen actively develops from the flowering stage and affects both generative and vegetative plant organs. Initial disease symptoms appear after flowering and are characterized by unexpected reddening, wilting, and dark necrotic lesions on flowers (Fig. 2).
 | Figure 2. Apricot blossoms infected with monilial blight |
 | Figure 3. Almond fruits infected with monilial blight |
|
4. Conclusions
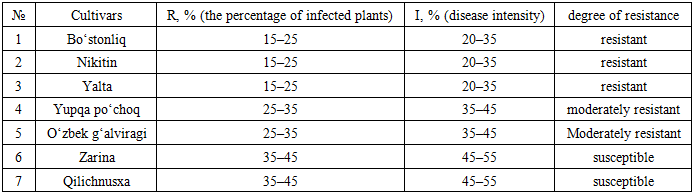
- Based on the results of field and laboratory studies, it can be concluded that disease development varies among apricot, cherry, peach, and almond cultivars and is strongly influenced by climatic conditions, relative humidity, and cultivar-specific traits. In Uzbekistan, disease incidence generally ranges from 15% to 45%, although periods of high spring humidity can increase infection rates to 50–55%. Regions such as Samarkand and Namangan, characterized by moderate temperatures and high humidity, exhibit the highest disease prevalence, whereas dry and hot regions including Bukhara, Syrdarya, Navoiy, and Khorezm tend to restrict pathogen spread.Among the cultivars studied, apricot varieties such as “Shalax,” “Qora orik,” and “Gulungi luchchak,” as well as cherry varieties “Valeriy Chkalov” and “Savri Surkhoni,” showed high susceptibility, while cultivars including “Isfarak,” “Ruhi juvanov,” “Bahor,” “Sariq Drogana,” “Bostanliq,” and “Nikitin” exhibited relative resistance. For peaches, susceptibility is largely influenced by fruit surface morphology, with smooth-skinned nectarines being more prone to infection compared to hairy peaches.Disease development is most intense in spring and declines as fruits mature under high temperature and low humidity. Selecting resistant cultivars is a key strategy for managing brown rot in Uzbekistan. When combined with strict orchard sanitation measures – such as removal of mummified fruits, pruning of blighted twigs, and control of infected blossoms – resistant cultivars provide an effective foundation for disease control.Overall, understanding the genetic diversity and population structure of M. laxa is crucial for predicting outbreaks and designing sustainable management strategies. Regular monitoring of pathogen populations, including assessment of fungicide sensitivity, supports targeted fungicide rotation programs, reduces the risk of resistance development, and enhances long-term control of brown rot in stone fruit orchards.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML