Nargiza Eshmurodova
PhD., Associate Professor, Department of Ecology, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Nargiza Eshmurodova, PhD., Associate Professor, Department of Ecology, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents the results of a comprehensive study of algal communities (algocenoses) in the Sudoche Lake System. The research included ecological monitoring, an assessment of aquatic biodiversity, and an analysis of ecosystem structure under conditions of a pronounced water mineralization gradient. Particular attention was given to the taxonomic composition of species, their ecological characteristics, and the spatial and structural organization of algal communities.
Keywords:
Sudoche Lake System, Algae identification, Ecological monitoring, Oligotrophic conditions, Mesotrophic conditions
Cite this paper: Nargiza Eshmurodova, Ecological Aspects of Algal Communities in the Sudoche Lake System, International Journal of Genetic Engineering, Vol. 13 No. 0, 2025, pp. 251-256. doi: 10.5923/j.ijge.20251310.01.
1. Introduction
The stabilization of the ecological situation in the Aral Sea basin, the restoration of ecosystems, the improvement of water and land resource management methods in the Aral Sea region, as well as the resolution of pressing and insufficiently addressed socio-economic and medical issues, represent areas of significant scientific and practical importance.This study is, to a certain extent, aimed at contributing to the implementation of the objectives outlined in the Decree of the President of the Republic of Uzbekistan dated January 28, 2022, No. PF-60, "On the Development Strategy of New Uzbekistan for 2022 - 2026," as well as the Decree dated November 23, 2023, No. PF-199, Annex 8, "National Program for the Sustainable Development of the Aral Sea Region for 2024 - 2028, the Expanded Implementation of Innovations and Green Technologies," along with other regulatory and legal documents governing this area [14,15].Algal communities play a vital role in the functioning of aquatic ecosystems by contributing to primary production, nutrient cycling, and biodiversity formation. In highly mineralized lake systems, the structure and dynamics of phytoplankton are shaped by a range of abiotic factors, primarily salinity levels, ion composition, temperature, and hydrological conditions [12,13].According to several studies (Dyakov, 2001; Karamyshev, 2008; Utepbergenov et al., 2015), high salinity leads to a simplified phytoplankton composition dominated by eurythermal and eurybiontic forms, mainly from Cyanophyta, Bacillariophyta, and Euglenophyta. A general decline in biodiversity is observed, accompanied by the predominance of tolerant species capable of withstanding ionic and osmotic stress. These communities, shaped under broad ecological gradients, serve as sensitive indicators of environmental change [1,4,10].Thus, the study of algal communities in saline lakes provides insight into the adaptive strategies of algae under extreme conditions and helps assess the resilience of aquatic ecosystems to natural and anthropogenic pressures.
2. Materials and Methods
The conservation of biodiversity at various organizational levels remains a key challenge in modern ecology. Restoration within short timeframes is complicated by natural and anthropogenic factors acting directly or indirectly. While anthropogenic impact is often the main driver of degradation, compensatory trends in some local ecosystems have also been reported [3,5-9,12,13].Standard field and laboratory algological methods were applied. Seasonal water and phytoplankton samples were collected from multiple sites in the Sudochye lake system using a Ruttner sampler and a 25 µm plankton net. Water parameters-temperature, pH, mineralization, dissolved oxygen-were measured in situ with portable multiparameter instruments [5].Samples were preserved with 4% formalin and analyzed under light microscopy (×400-1000). Algal taxa identification followed standard floristic and taxonomic guides [2]. Quantitative analysis, including species counts and biomass estimation, was conducted using the Utermöhl method and a Nageotte counting chamber [11].Ecological structure was assessed via species richness, dominance index, and Shannon-Wiener diversity index (Shannon & Weaver, 1949). Trophic state and saprobity evaluations were based on indicator species analysis [8]. Data processing and statistical analyses employed PAST [6], Excel, and SPSS software [2,8].
3. Result and Discussion
Between 2019 and 2024, comprehensive studies were conducted on the Sudochye lake system, which receives inflow from the Amu Darya River through its delta into the southern part of the Aral Sea. The research aimed to investigate the hydrological and ecological features of this system using a basin-based approach, carry out taxonomic assessments of algal communities (algocenoses), and perform ecological monitoring of water bodies located in both the western and eastern sectors of the Aral Sea, followed by an evaluation of their ecological status.The fieldwork focused on the study of algocenoses, ecological monitoring, assessment of hydrobiont biodiversity, and analysis of the structure of ecological communities under conditions of a high mineralization gradient. Observations were carried out at the following key monitoring sites: I. Lake Akushpa, situated at an altitude of 130 meters above sea level, in a rugged area along the northern escarpment of the Ustyurt Plateau (coordinates: 43˚49ʹ723ʺ N, 58˚32ʹ134ʺ E); II. Lake Bolshoye Sudochye, covering a total area of 46,467 hectares, located at an altitude of 156 meters above sea level (coordinates: 43˚29ʹ N, 58˚31ʹ E); III. Lake Begdulla-Aydyn, located at an altitude of 109 meters above sea level (coordinates: 43˚07ʹ689ʺ N, 58˚56ʹ980ʺ E); IV. Lake Karateren, situated at an altitude of 123 meters above sea level (coordinates: 43˚58ʹ195ʺ N, 58˚53ʹ686ʺ E).These locations were selected based on their contrasting ecological and hydrological conditions, allowing for a comparative analysis of algal communities and environmental parameters across the lake system.Field investigations were carried out across multiple seasons, resulting in the collection of over 864 algological samples from various monitoring sites within the Sudochye lake system. The taxonomic composition of the algal flora was analyzed under laboratory conditions, leading to the identification of dominant taxa and the evaluation of their ecological characteristics.The seasonal dynamics of algal communities were strongly influenced by a combination of abiotic environmental factors. Among the most impactful were observed fluctuations in air and water temperature, weakly alkaline pH values ranging between 7.8 and 8.1, low water transparency (0.09-0.20 m), and unstable hydro-physical conditions such as depth variability, color, and odor of the water (Fig. 1). 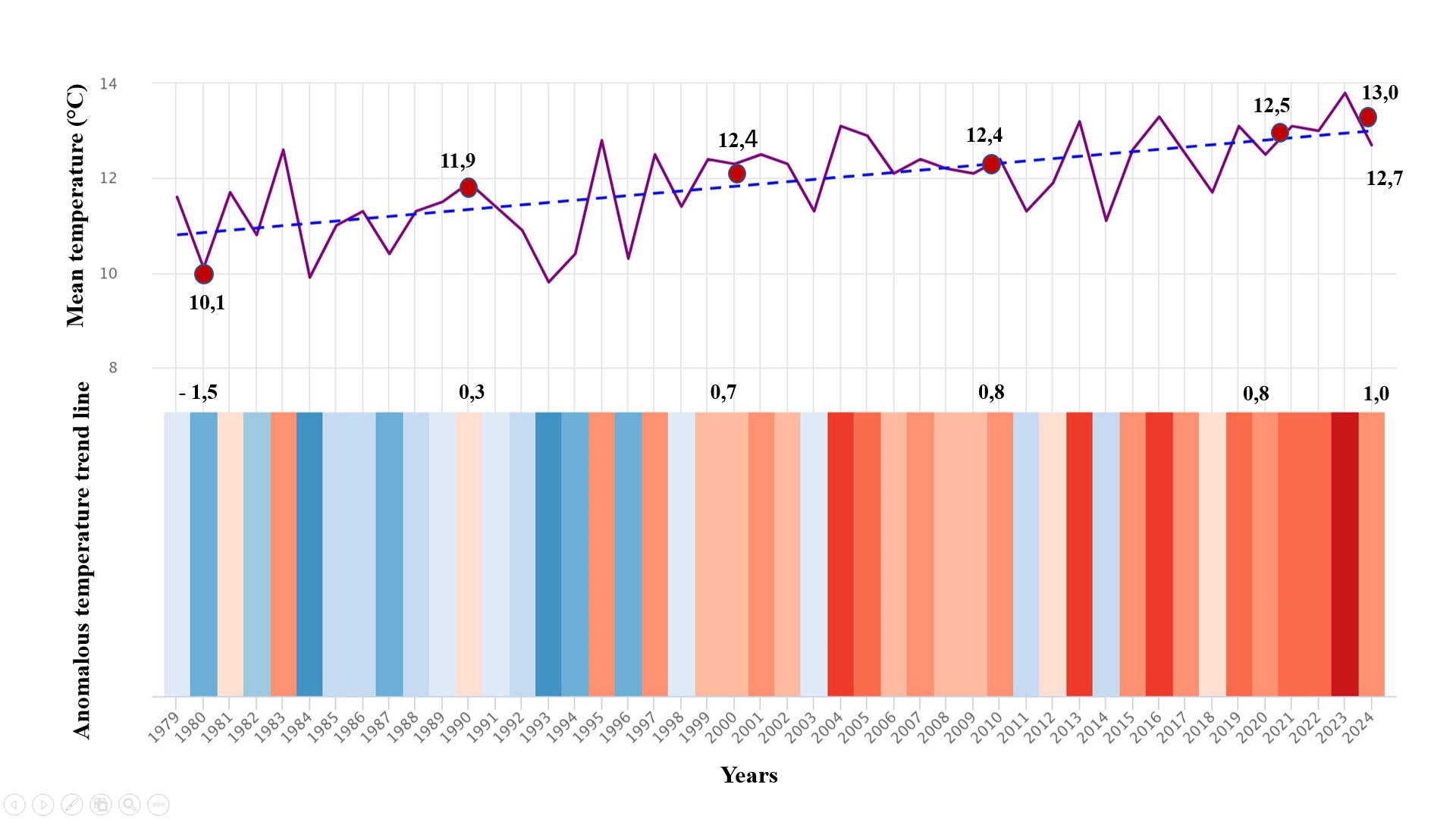 | Figure 1. Mean annual air temperature, temperature variability, and rates of temperature increase in the Sudochye lake system from 1980 to 2024 |
A clear pattern of seasonal and interannual variability in air temperature was recorded, with a consistent upward trend. By 2020, the mean annual air temperature reached 12.5 °C, reflecting an increase of 0.8 °C compared to previous baseline values. By 2024, this value rose to 12.7 °C, with a cumulative increase of 1.0 °C. The overall climatic trend projected a further rise towards 13.0 °C. These data indicate a steady year-on-year increase in air temperature over the past decade, contributing to changes in the thermal regime of the lake system and subsequently influencing the structure and seasonal succession of algal communities.Based on the analysis of long-term average monthly precipitation data, considering the trend indicated by the black lines, the highest precipitation levels are primarily observed during the spring period -in March, the summer-in June, and also in October, during the autumn season (Fig. 2).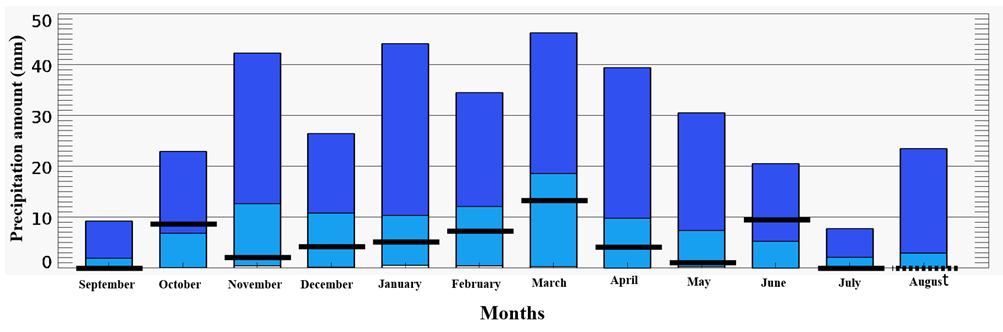 | Figure 2. Long-term mean monthly precipitation (mm) |
The analysis of long-term average monthly precipitation data revealed a pronounced seasonal unevenness in its distribution. The highest precipitation values were recorded mainly in the spring (March), summer (June), and autumn (October) months. In contrast, minimal precipitation was observed during certain months-such as September, May, and July-while in August, rainfall was nearly absent.These findings indicate the arid nature of the regional climate, particularly during the summer period. Such climatic conditions significantly influence the hydrological regime of the area and play a critical role in the formation and functioning of aquatic ecosystems.A comparative year-by-year analysis revealed significant hydrological changes in the Aral Sea region. By 2005, the sea level had dropped to an absolute elevation of N –30.33 m (a 25% decrease), while the volume of inflow from the Amu Darya distributaries declined to 8.40 km³ (a 20% reduction). The catchment area shrank to 19,600 km² (a 51.4% decrease), and the total water volume decreased to 125 km³ (a 66.7% reduction). Concurrently, water salinity levels increased sharply, reaching 90.0 g/L-an increase of 73.4%.By 2009, the situation had worsened: the sea level dropped further to N -27.53 m (a 32% decline), and the inflow volume fell to just 2.56 km³ (a 26% decrease). The catchment area reduced to 13,500 km² (a 64.6% decrease), and the total water volume decreased to 105 km³ (a 72% decline). At the same time, salinity levels peaked at 102 g/L, representing a 76.6% increase (see Figure 3).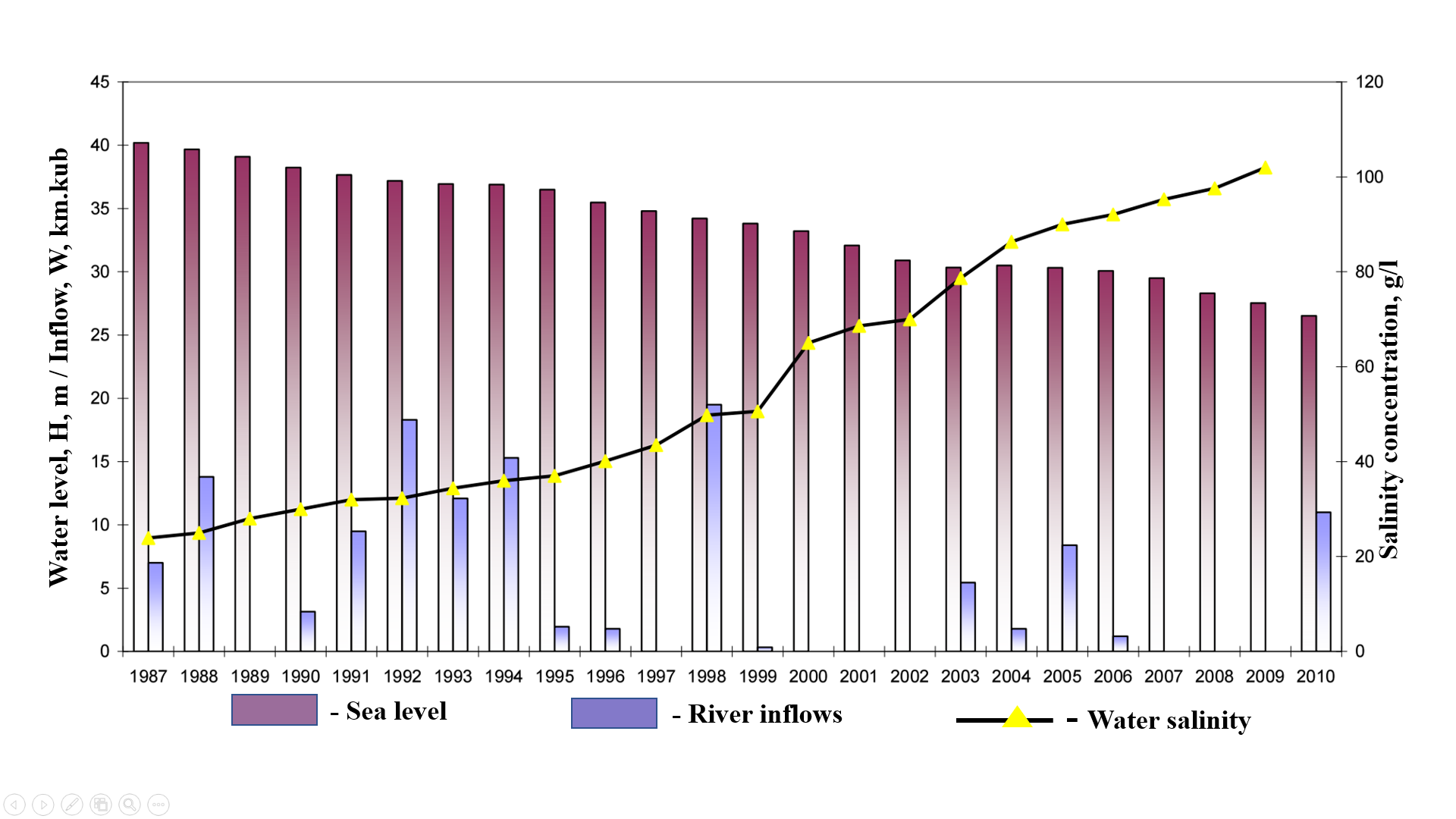 | Figure 3. Trends in water level, salinity, and river discharge in the Greater Aral Sea (1987-2010) |
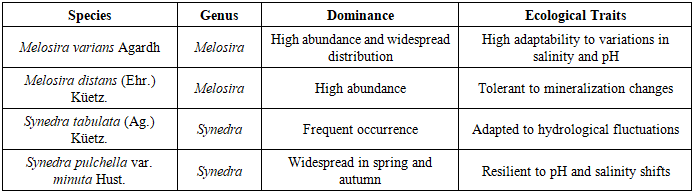
It was established that under conditions of elevated trophic levels and weak water circulation during the spring period, the development of diatoms (Bacillariophyta) and cyanobacteria (Cyanophyta) is notably intensified. These groups exhibit high tolerance to fluctuations in temperature and salinity. In particular, in areas characterized by low water transparency and high concentrations of suspended particles, a clear dominance was observed of species such as Melosira varians, Synedra tabulata, Fragilaria crotonensis, along with filamentous forms of cyanobacteria.Algal taxa with broad ecological plasticity-especially representatives of the genera Synedra, Melosira, Aphanizomenon, and Oscillatoria-demonstrated competitive advantages in both eutrophic and mesotrophic water bodies that experience seasonal thermal and light variability. The physico-chemical characteristics of the aquatic environment act as key ecological drivers shaping both the qualitative and quantitative composition of algal communities. These factors contribute to the formation of stable algocenoses that are well-adapted to the extreme and dynamic conditions of deltaic ecosystems.Analysis of hydrological parameters in the Sudochye lake system over the past five years revealed that the maximum surface water area was recorded in July 2020 (14,672.1 ha), followed by April 2021 (13,411 ha), June 2019 (12,977 ha), and October 2020 (12,276.3 ha).At the same time, the reduction in the surface area of the water body and the ongoing salinization processes in the Sudochye lake system have contributed to increased amplitude of temperature fluctuations throughout the water column over the course of the year. These changes also led to shifts in the phases of the thermal regime. Such hydrological alterations had a significant impact on the species composition of the algal communities, influencing both seasonal dynamics and overall biodiversity patterns.Under conditions of increased salinity and more pronounced temperature fluctuations, a marked restructuring of the algal communities was observed. There was a noticeable decline in the abundance and diversity of eurythermic and oligotrophic species, which typically thrive under relatively stable environmental conditions. Conversely, euryhaline and mesotrophic species capable of adapting to elevated mineral content and thermal stress showed significant growth.In particular, members of the genera Melosira, Synedra, and Fragilaria demonstrated high ecological plasticity and maintained stable populations despite environmental variability. In contrast, species sensitive to salinity and temperature fluctuations exhibited a decline in abundance or disappeared entirely from the algocenoses.These hydrological changes triggered substantial transformations in both the structural and functional characteristics of the aquatic ecosystems of Sudochye. The findings underscore the need for continued ecological monitoring and the development of adaptive strategies to preserve biodiversity and ensure the long-term resilience of these dynamic ecosystems.Changes in water availability, air temperature, and annual precipitation within the Sudochye lake system have led to significant transformations in aquatic ecosystems. The species composition of algal communities, their taxonomic structure, and trophic potential are directly influenced by these environmental factors.Under shifting climatic conditions, algal species with the highest levels of ecological adaptability have come to dominate the ecosystem. These species play a key role in shaping community structure and contribute to the overall resilience and functional stability of the aquatic ecosystem.Analysis of seasonally collected algological samples from the Sudochye lake system revealed that diatoms dominate the phytoplankton community, particularly species belonging to the classes Centrales and Pennatophyceae. Among these, the most represented orders are Discoidales, Cymbellales, Araphidales, and Raphidales.The taxonomic composition includes species from the families Coscinodiscaceae, Tabellariaceae, Fragilariaceae D.T., and Achnanthaceae, with a dominance of genera such as Melosira Agardh, Tabellaria Ehr., Synedra Ehr., Fragilaria Lyngb., and Achnanthes Bory.The species composition is characterized by significant diversity, with dominant diatom taxa including Melosira varians, Melosira distans, Synedra tabulata, and Synedra pulchella var. minuta. These species demonstrate a high degree of ecological plasticity and are well adapted to changing hydrochemical conditions, such as fluctuating salinity and pH levels (table 1).Table 1. Dominant Diatom Species in the Sudochye Lake System and Their Ecological Characteristics
 |
| |
|
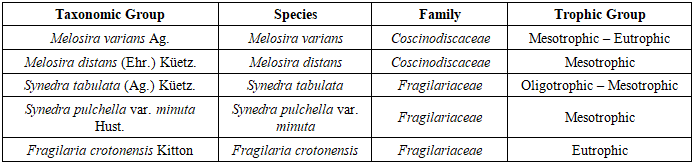
The dominant diatom species within the algal communities of the Sudochye lake system exhibit a broad range of ecological preferences. Some taxa are oligotrophic, favoring environments with low mineralization levels, while others are mesotrophic or eutrophic, showing resilience to elevated organic matter concentrations and variable environmental conditions. This taxonomic composition reflects the cumulative influence of multiple abiotic factors, including water temperature, transparency, depth, concentrations of dissolved substances, and seasonal climatic fluctuations.In addition, members of the families Fragilariaceae and Achnanthaceae play a significant role in shaping the ecological structure of the aquatic ecosystems, serving as important bioindicators of water quality. The species composition of the algal communities (algocenoses) displays notable seasonal variation, closely linked to changes in hydrological and environmental conditions.The high species diversity and the presence of ecologically heterogeneous taxa in the Sudochye lake system reflect complex adaptive strategies of algae to fluctuating environmental conditions. This functional diversity contributes to the overall stability and resilience of the ecosystem under dynamic and stress-prone circumstances. Trophic classification is based on known ecological preferences regarding nutrient availability and tolerance to salinity and organic enrichment (Table 2).Table 2. Dominant Diatom Species and Their Trophic Groups in the Sudochye Lake System
 |
| |
|
Based on their ecological characteristics, Melosira varians Ag. is classified as a brackish-water (oligobeta-mesosaprobic) diatom species and can serve as an indicator of declining water levels. Meanwhile, Melosira distans (Ehr.) Küetz., representing brackish and euryhaline (xeno-oligosaprobic) waters, signals periods of lowered water surface levels. The dominance of these species reflects hydrological changes within the Sudoche system and allows for assessment of water regime dynamics, which is crucial for ecological monitoring and regional water resource management. According to the saprobic index and salinity tolerance, euryhaline to mesohalobic species such as Synedra pulchella var. minuta Hust. (2.2), Synedra tabulata (Ag.) Küetz. (2.7), and Synedra ulna (Nitzsch) Ehr. (1.95) are capable of withstanding significant fluctuations in water mineralization across a wide range. Species like Achnanthes linearis var. cryptocephala (0.4) and Melosira distans (Ehr.) Küetz. (0.5), classified as xeno-oligosaprobic organisms, along with Achnanthes lanceolata (Bréb.) Grun. (0.75), a xeno-beta-mesosaprobic species, can serve as model indicators of high and variable mineralization levels in the Sudoche water bodies. The rapid adaptation and prolonged survival of species with diverse saprobic statuses — such as Synedra ulna (Nitzsch) Ehr. (1.95, beta-mesosaprobic), Melosira varians Ag. (1.85), and Achnanthes minutissima var. cryptocephala (1.45, oligo-beta-mesosaprobic) — enable predictions regarding the future biological diversity in these aquatic ecosystems.
4. Conclusions
Changes in the water regime of the Sudochye lake system, along with fluctuations in air temperature, precipitation, and anthropogenic impacts, have significantly affected aquatic ecosystems. These factors influence the composition, diversity, and trophic potential of algal communities.Species shifts in algocenoses are mainly driven by physical parameters such as light availability, air temperature (+1.5 °C to +27.5 °C), water temperature (+4.0 °C to +11.0 °C), transparency (0.12-0.20 m), turbidity, and pH (7.82–8.07). Morpho-edaphic conditions, including depth (0.5-1.15 m), surface area, and mineral-organic content, also shape internal ecosystem dynamics.Certain diatoms like Synedra pulchella var. minuta and Synedra tabulata demonstrate high ecological tolerance, indicating strong adaptability to unstable deltaic conditions.Long-term data show a steady rise in mean annual air temperature, with peak precipitation in March, June, and October. Between 1979 and 2024, annual precipitation averaged 113.2 mm, with anomalies up to 24.1 mm.
References
| [1] | Dyakov, Y. P. (2001). Ecological characteristics of phytoplankton in saline water bodies of Central Asia (pp. 45–62). Tashkent: FAN Publishing. |
| [2] | Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, 4(1), 9. https://palaeo-electronica.org/2001_1/past/past.pdf. |
| [3] | Johnson, M., & Lee, S. (2020). Compensatory dynamics in aquatic ecosystems under anthropogenic stress. Ecological Research, 35(4), 567–579. https://doi.org/10.1007/s11284-020-01763-8. |
| [4] | Karamyshev, F.R. (2008). Structural and Functional Features of Algal Communities in Highly Mineralized Lakes of the Aral Sea Region. Nukus: Karakalpak Branch of the Academy of Sciences. -Р. 88-104. |
| [5] | Komárek, J., & Anagnostidis, K. (1998). Cyanoprokaryota. 1. Teil: Chroococcales. In H. Ettl, G. Gärtner, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa (Vol. 19/1). Stuttgart: Gustav Fischer Verlag. |
| [6] | Krammer, K., & Lange-Bertalot, H. (1986–1991). Bacillariophyceae (Vols. 2/1–2/4). In H. Ettl, J. Gerloff, H. Heynig, & D. Mollenhauer (Eds.), Süßwasserflora von Mitteleuropa. Stuttgart: Gustav Fischer Verlag. |
| [7] | Pantle, R., & Buck, H. (1955). Die biologische Überwachung der Gewässer und die Darstellung der Ergebnisse. Gas- und Wasserfach: Wasser-Abwasser, 96, 604–620. |
| [8] | Shannon, C. E., & Weaver, W. (1949). The mathematical theory of communication. Urbana, IL: University of Illinois Press. |
| [9] | Smith, J., Brown, A., & Wilson, P. (2018). Factors influencing biodiversity restoration in freshwater ecosystems. Journal of Environmental Biology, 39(3), 215–223. |
| [10] | Utepbergenov, S. A., Rakhmanova, M. K., & Yusupov, R. K. (2015). Phytoplankton diversity and ecological assessment of water bodies in the South Aral region. Ecological Journal of Uzbekistan, 4(2), 35–42. |
| [11] | Utermöhl, H. (1958). Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitteilungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie, 9, 1–38. |
| [12] | Scientific Information Center of the Interstate Commission for Water Coordination (SIC ICWC). (2018). The Aral Sea and the Priaralie: Summary of research conducted by the SIC ICWC on monitoring and analysis of the socio-economic and environmental situation in the region from 1994 to 2018. Tashkent: Complex Print Publishing House. |
| [13] | Egorov, A. N. (2021). Peculiarities of salt lake ecosystems. German International Journal of Modern Science, 9, 25–30. |
| [14] | Eshmurodova, N. Sh., Urinboev, I. Yu., & Fakhriddinova, Z. F. (2022). Hydrobiological characteristics of the algocenoses in the southern part of the Aral Sea and marine lakes under conditions of anthropogenic eutrophication. In Modern research in biology: Problems and solutions. Proceedings of the International Scientific-Practical Conference (Part II, pp. 219–222). |
| [15] | Eshmurodova, N. Sh., & Fakhriddinova, Z. F. (2022). Hydrobiological, algological, and ecological characteristics of Lake Sarbas. Ecology Bulletin, 1(1), 32–36. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
