-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(9): 235-241
doi:10.5923/j.ijge.20251309.12
Received: Aug. 28, 2025; Accepted: Sep. 23, 2025; Published: Sep. 29, 2025

Reduction of Multiple Pregnancies through the Implementation of Elective Single Embryo Transfer (eSET) in IVF Programs
Islambekova Maftuna Khamid kizi1, Mamatova Zulaykho Aminjanovna2
1Ph.D. Student, Departament of Biology and Ecology, National University of Uzbekistan, Tashkent, Uzbekistan
2Ph.D., Departament of Biology and Ecology, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Islambekova Maftuna Khamid kizi, Ph.D. Student, Departament of Biology and Ecology, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The conducted research complies with the ethical principles of the World Medical Association (Declaration of Helsinki) concerning research involving human subjects. This retrospective study did not involve any experiments, interventions, or manipulations that could affect human health. The analysis was based exclusively on previously collected clinical data and statistical processing, without disclosure of patients’ personal information. The choice of embryo transfer day in in vitro fertilization (IVF) is a critical determinant of treatment success. This study analyzed clinical outcomes associated with day 3 (cleavage stage) and day 5 (blastocyst stage) embryo transfers in Uzbekistan, a country where IVF practices have undergone substantial transformation between 2023 and the first three months of 2025. A retrospective analysis was performed on 180 women undergoing IVF treatment at one of Uzbekistan’s leading reproductive centers: 60 patients during the first three months of 2023, 60 patients during the first three months of 2024, and 60 patients during the first three months of 2025. The study evaluated changes in embryo transfer timing, the number of embryos transferred, and implantation rates. The proportion of blastocyst transfers increased from 35% in the first three months of 2021 to 96% in early 2025, while cleavage-stage transfers declined from 65% to 4%. Implantation rates for day 5 transfers consistently exceeded those for day 3 transfers (75.0% vs. 59.3% on average). At the same time, the rate of elective single embryo transfer (eSET) rose from 24% in 2023 to 62% in 2025, thereby reducing the risk of multiple pregnancies. These findings highlight a significant transformation in IVF practice in Uzbekistan, aligning with international guidelines that prioritize blastocyst transfer and eSET. Such changes have led to improved implantation outcomes and reduced perinatal risks, reflecting a progressive transition toward more personalized and safer reproductive technologies.
Keywords: IVF, Blastocyst transfer, Cleavage-stage embryo, Embryo implantation, eSET, Uzbekistan, Assisted reproductive technology (ART), Embryo selection, Perinatal outcomes
Cite this paper: Islambekova Maftuna Khamid kizi, Mamatova Zulaykho Aminjanovna, Reduction of Multiple Pregnancies through the Implementation of Elective Single Embryo Transfer (eSET) in IVF Programs, International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 235-241. doi: 10.5923/j.ijge.20251309.12.
1. Introduction
- The optimal timing of embryo transfer (day 3 cleavage stage vs day 5 blastocyst stage) remains a pivotal clinical decision in IVF, with direct implications for implantation, clinical pregnancy, multiple pregnancy rates, and perinatal outcomes. Aggregate evidence from systematic reviews and meta-analyses indicates that blastocyst transfer is associated with higher clinical pregnancy and live birth rates per transfer, whereas effects on cumulative live birth rate (CLBR) are contingent on cycle cancellations, cryopreservation strategy, and patient case-mix [1,2,3,4].Mechanistically, the advantages of day-5 transfer are attributed to natural selection during extended culture (only the most developmentally competent embryos reach the blastocyst stage), improved morphologic and morphokinetic information for embryo selection, and potentially better embryo–endometrium synchrony within the implantation window; these factors underpin the rationale for elective single embryo transfer (eSET) when sufficient high-quality embryos are available, reducing multiple pregnancy risk without compromising cumulative outcomes [5,6,7,8,9,10].However, extended culture has limitations: a non-negligible fraction of embryos may arrest before blastulation (reported up to 30–50% in some series), notably among patients with diminished ovarian reserve or poor oocyte quality, increasing fresh-cycle transfer cancellations and affecting CLBR and cost-effectiveness; thus, cleavage-stage transfer remains clinically justified in such subgroups [11,12,13].Perinatal safety data are mixed: population studies and meta-analyses report modest increases in preterm birth and birth-weight differences after blastocyst transfer in certain contexts, but when controlling for fresh vs frozen transfer and other confounders these differences often attenuate; therefore, rigorous adjustment for clinical and laboratory variables is required when assessing perinatal risk [14,15,16,17].Technological variables (culture media, incubator systems, vitrification protocols, embryology laboratory expertise) significantly influence D5 outcomes — high-quality laboratory practice amplifies the benefits of blastocyst transfer. Adjunctive embryo selection tools (time-lapse imaging, PGT-A) may further refine eSET strategies, but their routine role and net impact on CLBR and long-term offspring health require continued evaluation [18,19,20,21]. Finally, guideline recommendations (ASRM, ESHRE) generally endorse eSET in appropriate patients, yet national adoption must reflect local demographics, resource availability, and economic considerations. In countries with developing IVF programs (including Uzbekistan), local CLBR and perinatal outcome data are essential to adapt international best practices to the national context [2,4,22].D5 transfer typically improves per-transfer pregnancy outcomes and facilitates safe implementation of eSET; nonetheless, individualized decision-making — accounting for embryo yield, patient prognosis, laboratory capacity, and regional factors — is required [22].
2. Materials and Methods
- This retrospective study analyzed in vitro fertilization (IVF) protocols performed at one of the leading reproductive centers in Uzbekistan between 2023 and 2025 (first three months of each year were included). The study population consisted of women of reproductive age undergoing infertility treatment via IVF. The parameters evaluated included embryo transfer timing (day 3 or day 5), number of embryos transferred, frequency of elective single embryo transfer (eSET), and implantation rates.1. Controlled Ovarian Stimulation (COS)Patients underwent ovarian stimulation to promote multifollicular growth within a single cycle. Recombinant follicle-stimulating hormone (r-FSH) and/or human menopausal gonadotropin (hMG) were administered. Follicular dynamics were tracked via serial transvaginal ultrasound scans and serum estradiol (E2) assays. To avoid premature luteinizing hormone (LH) peaks, gonadotropin-releasing hormone (GnRH) agonists or antagonists were introduced.2. Ovulation InductionFinal oocyte maturation was triggered using either human chorionic gonadotropin (hCG) or a GnRH agonist, depending on the assessed risk of ovarian hyperstimulation syndrome (OHSS). Oocyte retrieval was performed 34–36 hours later.3. Oocyte RetrievalFollicular aspiration was carried out under transvaginal ultrasound guidance using a specialized aspiration needle and intravenous sedation. Collected oocytes were immediately transported to the embryology laboratory.4. Oocyte Maturity EvaluationEach oocyte was examined under an inverted microscope to determine its nuclear status. Only mature oocytes at the metaphase II (MII) stage, identified by the presence of the first polar body, were selected for insemination.5. Semen ProcessingSemen samples were prepared by density gradient centrifugation and/or swim-up. In azoospermic cases, spermatozoa were surgically obtained (PESA, TESA, or micro-TESE) and subsequently processed.6. Fertilization ProceduresDepending on clinical indications, two fertilization approaches were applied:• Conventional IVF: co-incubation of mature oocytes with spermatozoa for 16–18 hours;• Intracytoplasmic Sperm Injection (ICSI): performed in cases of male infertility or previous IVF failure.7. Embryo CultureZygotes were maintained in specialized sequential media under controlled culture conditions (5% O₂, 6% CO₂, 37°C). Embryos were cultured either up to the cleavage stage (Day 3) or extended to the blastocyst stage (Day 5–6), depending on their developmental potential and the clinical plan.8. Embryo EvaluationEmbryos were examined daily. Cleavage-stage embryos were graded according to cell number, blastomere symmetry, degree of fragmentation, and multinucleation. Blastocyst grading followed Gardner’s criteria (expansion, inner cell mass, trophectoderm).9. VitrificationSurplus viable embryos not transferred were cryopreserved using vitrification in liquid nitrogen (–196 °C) with cryoprotectants (dimethyl sulfoxide, ethylene glycol). These embryos were later used in frozen-thawed cycles.10. Embryo Transfer (ET)ET was carried out under ultrasound guidance using a soft catheter. Both fresh and vitrified-thawed embryos were transferred. The transfer stage (Day 3 or Day 5) was determined by embryo quality and number. Elective single embryo transfer (eSET) was prioritized when high-quality blastocysts were available.11. Luteal Phase SupportAfter ET, progesterone supplementation (oral, vaginal, or intramuscular) was prescribed until pregnancy confirmation and continued up to 10–12 weeks of gestation if successful.12. Pregnancy AssessmentSerum β-hCG testing was performed 9–12 days after ET. If positive, transvaginal ultrasound was conducted 5–7 days later to confirm intrauterine pregnancy.13. Study GroupsParticipants were retrospectively classified into two groups according to the stage of embryo transfer:• Group 1: cleavage-stage transfers (Day 3);• Group 2: blastocyst transfers (Day 5).Additionally, temporal trends in embryo transfer practice were evaluated, reflecting the gradual shift toward international recommendations and the promotion of eSET to minimize multiple pregnancy rates.
3. Results and Discussion
- A retrospective analysis of embryo transfers performed in our clinic between 2022 and 2025 demonstrated a clear trend toward an increasing proportion of blastocyst-stage transfers, accompanied by a corresponding decline in Day 3 transfers.In 2022, the majority of embryo transfers were carried out at the cleavage stage (35%), while blastocyst-stage transfers accounted for 65%. By 2023, a substantial shift was observed, with blastocyst transfers rising to 95%, reflecting a decisive move toward later-stage transfer in clinical practice. This upward trend continued in subsequent years, reaching 96% in 2024 and 97% in 2025. As a result, Day 3 transfers progressively decreased, representing only 3% of all embryo transfers by 2025.Taken together, these results demonstrate a near-complete transition from cleavage-stage to blastocyst-stage transfer between 2022 and 2025, in line with global trends aimed at enhancing IVF efficiency and improving reproductive outcomes (Figure 1).
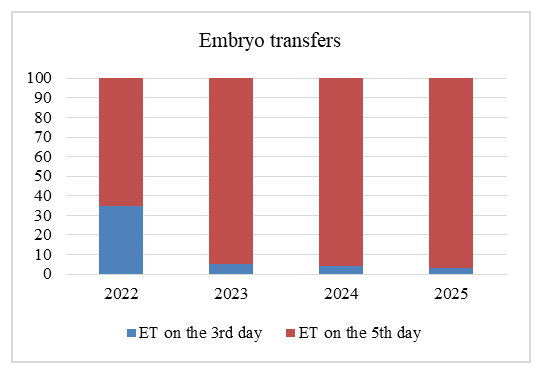 | Figure 1. Frequency of Day 5 (blastocyst) and Day 3 (cleavage stage) embryo transfers |
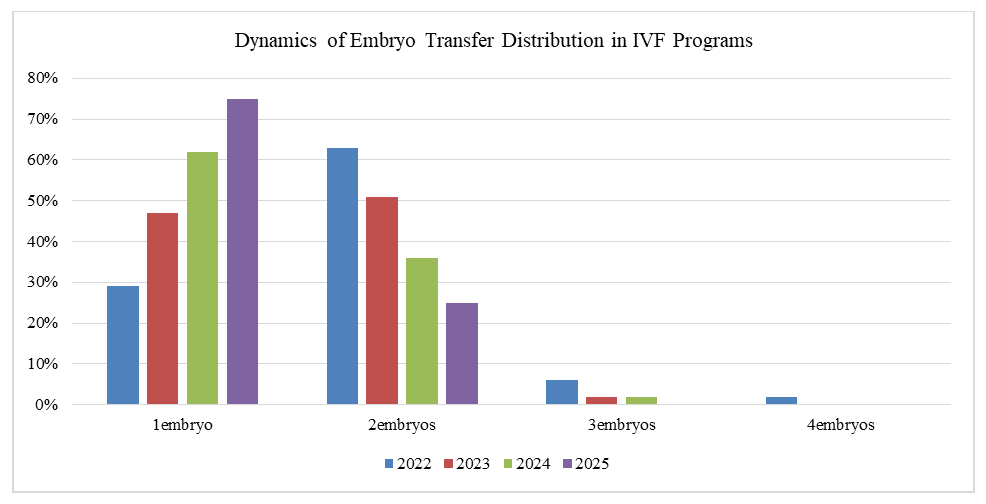 | Figure 2. Trends in the frequency of single, double, and multiple embryo transfers from 2022 to 2025 |
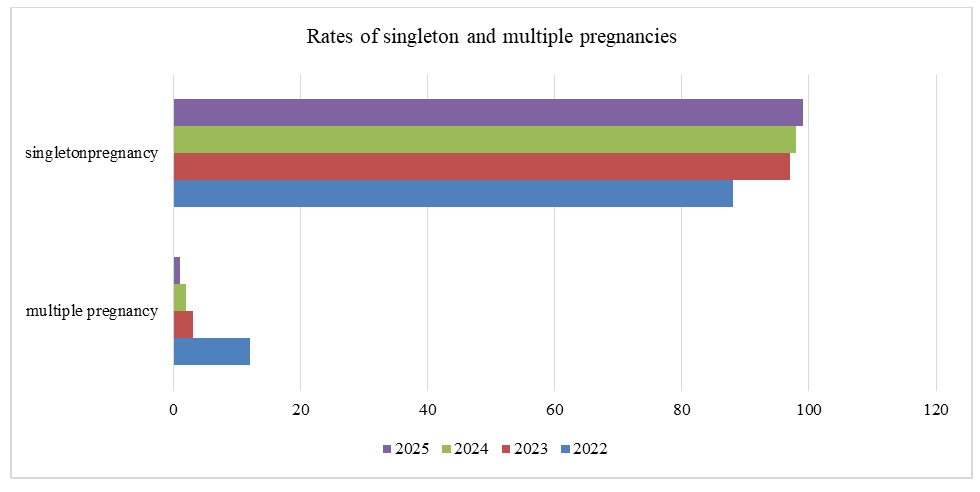 | Figure 3. Rates of singleton and multiple pregnancies among couples undergoing IVF from 2022 to 2025 |
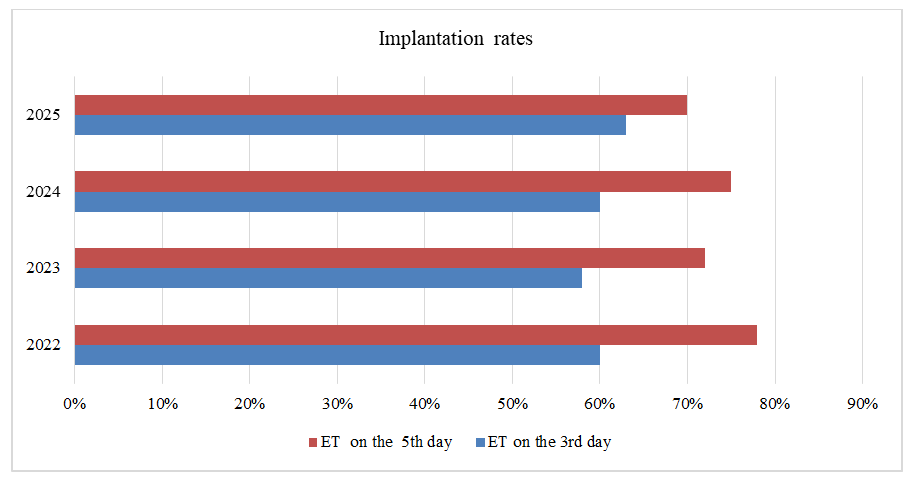 | Figure 4. Implantation rates of embryos transferred at the cleavage stage (Day 3) and blastocyst stage (Day 5) |
4. Conclusions
- The results of this study highlight substantial progress in embryo culture and transfer strategies within assisted reproductive technology (ART) programs. The progressive decline in Day 3 cleavage-stage transfers and the transition toward Day 5 blastocyst-stage transfers reflect the integration of contemporary clinical standards aimed at improving both the efficacy and safety of IVF procedures.Culturing embryos to the blastocyst stage enables more precise embryo selection, as only those with high developmental competence progress to this stage, which strongly correlates with both morphological and genetic quality. The transfer of such embryos significantly enhances implantation and pregnancy rates, while reducing the need for multiple transfers and minimizing the risk of multiple gestations—particularly when combined with elective single embryo transfer (eSET) strategies.International recommendations (ESHRE, ASRM, Istanbul Consensus) emphasize the major advantages of blastocyst-stage transfer, including:• improved cycle efficiency through enhanced embryo selection [Alpha Scientists in].• better synchronization of the endometrial implantation window with embryonic development.• the possibility of performing preimplantation genetic testing (PGT).• reduction in the number of failed cycles.The reduction of Day 3 transfers to only 4% by 2024 reflects the maturity of the laboratory environment, advancements in culture conditions, and optimized embryological logistics. This transformation underscores the trend toward individualized treatment, minimizing reproductive risks, and achieving consistent clinical outcomes even with single embryo transfer.In summary, the adoption of blastocyst-stage transfer not only mirrors global trends in ART but also serves as a reliable marker of embryological quality within a given center. The presented findings demonstrate our clinic’s strong alignment with international standards and its contribution to the optimization of both laboratory processes and clinical outcomes in infertility treatment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML