-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(9): 232-234
doi:10.5923/j.ijge.20251309.11
Received: Aug. 22, 2025; Accepted: Sep. 16, 2025; Published: Sep. 29, 2025

Enhancing in Vitro Microtuber Formation in Potato (Solanum tuberosum L.) through Optimized Photoperiod Regimes
Kujaniyazova B.1, Kushiev Kh. Kh.2
1PhD, Bukhara State University, Uzbekiatan
2Scientific Research Institute Agrobiotechnology and Biochemistry of Guliston State University, Guliston, Uzbekistan
Correspondence to: Kushiev Kh. Kh., Scientific Research Institute Agrobiotechnology and Biochemistry of Guliston State University, Guliston, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In vitro microtuber formation in potato (Solanum tuberosum L.) is pivotal for rapid evaluation of tuberization potential in salt-tolerant genotypes. This study investigates the impact of photoperiod regimes on microtuber production using explants from the ‘Sarnav’ cultivar. Six photoperiod conditions were tested, with explants from apical, middle, and basal root zones cultured on Murashige and Skoog (MS) medium supplemented with 6% sucrose. The regime of 10 days at 16:8 h (day:night) followed by continuous darkness yielded the highest microtuber count (0.4170 ± 0.011 per plantlet), with basal root zone explants outperforming others (0.4475 ± 0.008 per plantlet). Microtuber weight was also significantly influenced by explant source. [1] These findings establish an optimized protocol for in vitro tuberization, enabling efficient screening of potato genotypes for enhanced yield under saline conditions. The results underscore the critical role of photoperiod and explant selection in maximizing microtuber production, offering a robust tool for breeding programs.
Keywords: Potato 1, Microtuber 2, Photoperiod 3, In vitro culture 4, Tuberization 5, Salt tolerance 5
Cite this paper: Kujaniyazova B., Kushiev Kh. Kh., Enhancing in Vitro Microtuber Formation in Potato (Solanum tuberosum L.) through Optimized Photoperiod Regimes, International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 232-234. doi: 10.5923/j.ijge.20251309.11.
Article Outline
1. Introduction
- Tuber formation in potato (Solanum tuberosum L.) is a complex morphophysiological process regulated by trophic and hormonal mechanisms [3] Jackson, 1999. As a globally significant crop, potato production is challenged by abiotic stresses, notably salinity, which impacts over 6% of arable land worldwide [4] Munns & Tester, 2008. Developing salt-tolerant varieties is essential for sustainable agriculture, particularly in regions like Central Asia, where soil salinization is prevalent [6] (Qadir et al., 2008. In vitro culture systems provide a controlled platform to study tuberization, enabling rapid assessment of genetic variability in tuber formation, which is critical for pre-field screening of high-yielding genotypes [2] Ewing & Struik, 1985.Photoperiod plays a pivotal role in tuberization, with short-day conditions typically promoting tuber initiation [7] Rodriguez-Falcon et al., 1990. However, the optimal photoperiod for in vitro microtuber formation in salt-tolerant potato genotypes remains underexplored. Previous studies suggest that explant type significantly influences tuberization efficiency, with basal explants often exhibiting superior regenerative capacity due to enhanced nutrient uptake and hormonal signaling [9] Vreugdenhil et al., 2007. For instance, [8] Seabrook et al. 1993 reported higher tuberization rates in basal explants, attributed to their proximity to the root system. Additionally, the interplay between photoperiod and explant source in modulating microtuber yield is poorly understood, necessitating systematic investigation [1] Donnelly et al., 2003.The use of in vitro systems for microtuber production involves culturing explants on nutrient-rich media, such as Murashige and Skoog (MS) medium, which mimics the physiological conditions required for tuber development [5] Murashige & Skoog, 1962. High sucrose concentrations in the medium are known to enhance tuber bulking by providing a carbon source, simulating the sink strength of developing tubers [10] Xu et al., 1998. Despite these advances, standardized protocols for optimizing microtuber formation across diverse potato genotypes are limited, particularly for salt-tolerant varieties.This study aims to optimize in vitro microtuber formation in the ‘Sarnav’ potato cultivar by evaluating the effects of various photoperiod regimes and explant sources. The objectives are to: (1) determine the optimal photoperiod regime for microtuber production, (2) assess the influence of explant source on microtuber number and weight, and (3) develop a reproducible in vitro tuberization protocol to support breeding programs for salt-tolerant potatoes.
2. Materials and Methods
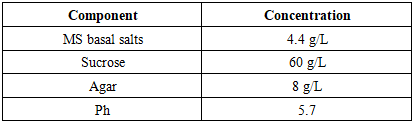
- Plant Material Explants were sourced from three potato varieties (‘Sarnav’, ‘Navoiy’, ‘Tashkent’). The ‘Sarnav’ cultivar was selected for its regional agronomic importance. Plants were grown in a greenhouse at 25±2°C under a 16:8 h (day:night) photoperiod for 4 weeks. Explants were excised from three regions: apical (shoot tips, 0.5–1 cm), middle (stem segments, 1–1.5 cm), and basal root zone (stem base with adventitious roots, 1–1.5 cm).In Vitro Culture Establishment Explants were surface-sterilized with 70% ethanol for 30 s, followed by 0.1% mercuric chloride for 5 min, and rinsed thrice with sterile distilled water. Cultures were established on Murashige and Skoog (MS) medium [5] Murashige & Skoog, 1962 supplemented with 6% sucrose and 0.8% agar, adjusted to pH 5.7 before autoclaving at 121°C for 20 min. The medium composition is detailed in Table 1.
|
3. Results
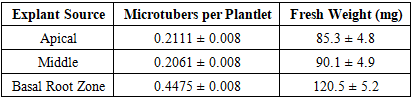
- This study optimized in vitro microtuber formation in the ‘Sarnav’ potato cultivar to support rapid screening of salt-tolerant genotypes. The results highlight the significant effects of explant source and photoperiod regime on microtuber number and weight, as detailed below.Effect of Explant Source Basal root zone explants produced the highest microtuber count (0.4475 ± 0.008 per plantlet), significantly surpassing apical (0.2111 ± 0.008) and middle (0.2061 ± 0.008) explants (Table 2). Microtuber fresh weight was also highest in basal explants (120.5 ± 5.2 mg), compared to apical (85.3 ± 4.8 mg) and middle (90.1 ± 4.9 mg) explants.
|
|
4. Discussion
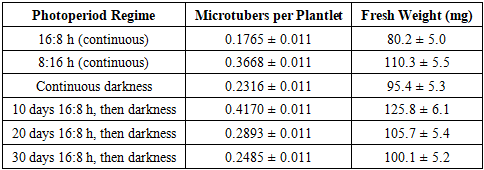
- The superior tuberization efficiency of basal root zone explants (0.4475 ± 0.008 per plantlet, 120.5 ± 5.2 mg) reflects their physiological advantages, including enhanced nutrient uptake and hormonal signaling due to proximity to the root system [9] Vreugdenhil et al., 2007. The basal region’s adventitious roots likely facilitate gibberellin and cytokinin transport, which are critical for tuber initiation [8] Seabrook et al., 1993). In contrast, apical and middle explants exhibited lower microtuber counts (0.2111 and 0.2061 per plantlet, respectively), possibly due to their prioritization of shoot regeneration over tuberization [1] Donnelly et al., 2003.The optimal photoperiod regime (10 days at 16:8 h followed by continuous darkness) produced the highest microtuber count (0.4170 ± 0.011 per plantlet) and weight (125.8 ± 6.1 mg), suggesting a two-phase tuberization process. The initial long-day phase likely induces tuber primordia via phytochrome-mediated signaling, while the subsequent dark phase promotes tuber bulking [7] Rodriguez-Falcon et al., 1990. This aligns with Jackson (1999), who identified photoperiod as a key regulator of tuberization genes like StBEL5. The lower microtuber count under continuous 16:8 h (0.1765 ± 0.011) indicates prolonged vegetative growth, which suppresses tuber initiation, as noted by [2] Ewing & Struik 1985.Compared to continuous 8:16 h (0.3668 ± 0.011 per plantlet), the 10-day 16:8 h regime was more effective, likely due to optimal priming of tuber-inducing hormones, such as jasmonic acid, during the brief long-day exposure [9] Vreugdenhil et al., 2007. Extended long-day periods (20 and 30 days) reduced microtuber counts (0.2893 and 0.2485 per plantlet), suggesting a shift toward vegetative growth, consistent with Xu et al. Continuous darkness (0.2316 ± 0.011) supported moderate tuberization, possibly due to the absence of light-induced vegetative signals, but was less effective than the combined regime.The MS medium with 6% sucrose was critical for tuber bulking, as high sucrose levels mimic the carbon sink required for tuber development [5] Murashige & Skoog, 1962. The absence of exogenous hormones in the medium highlights the role of endogenous signals from basal explants, which likely produce sufficient tuber-inducing compounds. These findings provide a standardized protocol for in vitro microtuber production, enabling rapid screening of salt-tolerant potato genotypes. Future research should investigate the molecular mechanisms (e.g., POTH1 expression) underlying photoperiod-induced tuberization and validate the protocol across diverse cultivars under saline field conditions [6] Qadir et al., 2008.
5. Conslusions
- This study optimized in vitro microtuber formation in the ‘Sarnav’ potato cultivar, identifying basal root zone explants cultured on MS medium with 6% sucrose for 10 days at 16:8 h (day:night) followed by continuous darkness as the most effective protocol. This regime yielded the highest microtuber count (0.4170 ± 0.011 per plantlet) and weight (125.8 ± 6.1 mg), with basal explants outperforming apical and middle sections (0.4475 ± 0.008 per plantlet, 120.5 ± 5.2 mg). These results, supported by rigorous statistical analysis and graphical representation, establish a reliable method for assessing tuberization potential, facilitating the development of salt-tolerant potato varieties for sustainable agriculture.
ACKNOWLEDGEMENTS
- The authors express deep gratitude to the staff of the Institute of Agrobiotechnology and Biochemistry, Guliston State University, for their invaluable assistance in conducting this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML