-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(9): 215-223
doi:10.5923/j.ijge.20251309.09
Received: Aug. 26, 2025; Accepted: Sep. 20, 2025; Published: Sep. 29, 2025

Preparation of a Biogel by Immobilizing TERIA S Bacterial Cells on CMC-Based Hydrogel and Its Effect on the Germination of Ferula Tadshikorum Seeds
Rajabov T.1, Tashmuxamedova Sh.2, Khalkuziyeva M.3, Khatamov D.4
1National University of Uzbekistan, Department of Biotechnology and Microbiology, PhD Student, Republic of Uzbekistan, Tashkent
2National University of Uzbekistan, Department of Biotechnology and Microbiology, Professor, Republic of Uzbekistan, Tashkent
3National University of Uzbekistan, Department of Biotechnology and Microbiology, Associate Professor, Republic of Uzbekistan, Tashkent
4National University of Uzbekistan, Department of Biotechnology and Microbiology, Assistant, Republic of Uzbekistan, Tashkent
Correspondence to: Rajabov T., National University of Uzbekistan, Department of Biotechnology and Microbiology, PhD Student, Republic of Uzbekistan, Tashkent.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Currently, the demand for medicinal plants is steadily increasing; therefore, their use as a source of raw materials has significant economic potential. A number of medicinal plants are endemic, and they can serve as valuable sources for the development of biopharmaceuticals with global market relevance. However, environmental changes, depletion of macro- and microelements in soils, and, most importantly, water scarcity negatively affect agricultural crops, including medicinal plants. These factors reduce the yield and biological activity of pharmacologically important compounds. In this study, an eco-friendly hydrogel was synthesized using carboxymethylcellulose (CMC) derived from cellulose waste. The hydrogel is biodegradable, non-polluting to soil, and possesses high water retention capacity. Its physicochemical properties were investigated, and pore sizes were determined using scanning electron microscopy (SEM) along with elemental composition analysis. TERIA S bacterial cells were immobilized within the hydrogel pores. To confirm immobilization, the obtained biogel was incubated at 50°C for 10 days, followed by cultivation in nutrient medium, yielding positive results. The resulting biogel was further applied for encapsulation of Ferula tadshikorum seeds to evaluate its effect on germination, growth, and development. Experimental results showed that seeds encapsulated in the biogel demonstrated higher germination rates, enhanced root system activity, and improved uptake of essential nutrients such as phosphorus, potassium, calcium, silicon, sulfur, and nitrogen. Furthermore, this approach reduced the plant’s water demand, promoted growth and development, increased resistance to phytopathogens, and improved both yield and quality of the medicinal plant. Field experiments were conducted on rain-fed lands in the Surkhandarya region.
Keywords: Hydrogel, Biogel, Immobilization, TERIA S, Ferula tadshikorum, Seed germination, Biotechnology, Medicinal plants
Cite this paper: Rajabov T., Tashmuxamedova Sh., Khalkuziyeva M., Khatamov D., Preparation of a Biogel by Immobilizing TERIA S Bacterial Cells on CMC-Based Hydrogel and Its Effect on the Germination of Ferula Tadshikorum Seeds, International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 215-223. doi: 10.5923/j.ijge.20251309.09.
1. Introduction
- Currently, global climate change, rapid population growth, and the expansion of agricultural activities have led to a sharp increase in the demand for water resources. According to the World Health Organization and the United Nations Economic Commission for Europe, more than 70% of the world’s freshwater consumption is used in agriculture, particularly for irrigation purposes [1]. At the same time, reserves of drinking and irrigation water are steadily declining, which poses serious threats to food security, ecosystem stability, and human health [2]. Water scarcity is especially acute in semi-arid and arid regions. Under such conditions, the rational use of water in agriculture, particularly in the cultivation of medicinal plants, the application of effective agrotechnical practices, and the development of new biotechnological approaches have become urgent tasks [3].Reducing the water demand of plants while preserving or even enhancing their medicinal properties is one of the most important scientific directions. In particular, hydrogels derived from cellulose, bacterial biopreparations, and biogel technologies are considered innovative solutions that enable the successful cultivation of medicinal plants under limited water supply. Such approaches contribute to sustainable productivity by retaining soil moisture for extended periods, protecting plants from stress conditions, and improving nutrient uptake [4]. From this perspective, cultivating medicinal plants such as Ferula tadshikorum using biogel technologies not only ensures ecological sustainability and water conservation but also plays a vital role in meeting the demand for pharmaceuticals while protecting the environment. Over the past 30 years, extensive scientific developments have focused on the synthesis of hydrogels and their application in agriculture. The term “hydrogel” originates from the Greek words “hydro” (water) and “gel” (a substance between solid and liquid states), meaning “water-containing gel.” It was first introduced in the 1960s by Otto Wichterle and Drahoslav Lim, who succeeded in creating a crosslinked polymer—hydrogel—based on poly(2-hydroxyethyl methacrylate) (polyHEMA), capable of absorbing water [5].Natural hydrogels contain biopolymers such as cellulose, starch, alginate, chitosan, and gelatin, which are considered environmentally safe due to their biodegradability. These hydrogels are suitable for use in sustainable agricultural practices [7]. For instance, cellulose-based hydrogels have been shown to increase soil water retention and improve crop productivity. Similarly, chitosan-based hydrogels, derived from crustacean shells, are widely applied due to their antimicrobial activity and biodegradability, making them effective in protecting plants against harmful microorganisms [8]. However, natural hydrogels typically exhibit lower mechanical strength and water retention capacity compared to synthetic hydrogels, limiting their application under complex agroecological conditions [9,10].In Uzbekistan, the biology and natural reserves of plants belonging to the genus Ferula have been studied by U. Rakhmonqulov (1981; 1999), S. Meliboev (1985), Kh. Nishonboeva (1972), I.U. Muqumov (1993), and Kh. Rakhmonov (2017), as well as O. Avalboev (2020). The chemical and pharmacological properties of Ferula species were investigated by A.I. Saidkhodjaev (1984), G.K. Nikonov (1971), and V.M. Mlikov (1998), while pharmacological effects were assessed by Rakhimov (2007). According to U. Rakhmonqulov (1999) and S. Meliboev, more than 20 species of Ferula have been identified in natural reserves. In Uzbekistan, M.A. Khalkuziyeva (2022) carried out studies on the prospects of establishing Ferula tadshikorum plantations in rain-fed lands, while A.E. Sharipov (2025) also contributed to research in this field.At present, in-depth investigation of the ontogenetic biological characteristics of these species, the establishment of plantations for resin (gum) production, and the rational use of existing natural habitats remain among the most pressing scientific and practical tasks.
2. Materials and Methods
- In the course of this study, a hydrogel was synthesized based on carboxymethyl cellulose (CMC). To investigate the microstructural and morphological characteristics of the synthesized hydrogel, Scanning Electron Microscopy (SEM) was employed. This method enabled the determination of pore size, shape, and distribution within the hydrogel matrix. However, since SEM operates under vacuum conditions and uses an electron beam, direct observation of hydrogels with high moisture content was not feasible. Therefore, specific sample preparation steps were performed prior to SEM analysis.First, the hydrogel samples were thoroughly dried using one of two methods: vacuum drying or lyophilization (freeze-drying). For SEM analysis, lyophilization was considered the most suitable method, as it removed water while preserving the microstructure of the hydrogel. In this process, hydrogel samples were frozen at −20°C to −80°C and then subjected to sublimation in a freeze-dryer [11]. As a result, water crystals sublimated without damaging the structural integrity of the hydrogel.The dried samples were cut into small pieces (approximately 5–10 mm) and mounted on SEM stubs. Since hydrogels are dielectric materials and non-conductive, their surfaces were coated with a conductive layer of gold (Au), platinum (Pt), or palladium (Pd) using a sputter-coating apparatus. The metal coating thickness was approximately 5–10 nm, which ensured uniform electron beam distribution and improved image resolution.The coated samples were placed in the SEM chamber. The accelerating voltage, one of the main operating parameters, was adjusted within the range of 5–20 kV. Additional parameters such as focus, brightness, contrast, and spot size were optimized according to image quality. After proper calibration, high-resolution SEM images were obtained, allowing for the characterization of pore diameters, internal structural uniformity, and bacterial cell localization within the hydrogel [12]. These analyses provided critical insights into the physicochemical and morphological properties of the hydrogel. In the subsequent experiments, Teria-S biofertilizer bacteria were immobilized within the CMC-based hydrogel. For this purpose, 100 ml of Eschbi nutrient medium was inoculated with 2.0 ml of Teria-S biofertilizer and cultivated under deep fermentation for three days. The resulting suspension was then introduced into hydrogel samples at concentrations of 1.0%, 2.0%, and 3.0%, followed by incubation for 12 to 60 hours under shaking conditions (180 rpm) at 30–32°C. The prepared biogel was then used for encapsulation of Ferula tadshikorum seeds. As the experimental object, Ferula tadshikorum—a highly valuable and widely cultivated medicinal plant—was selected due to its economic significance. F. tadshikorum is a perennial, monocarpic hemicryptophyte with a taproot system. The upper part of the root forms a thickened rhizome, measuring 30–40 cm in length, with a basal neck diameter ranging from 25 to 40 cm. The plant is characterized by a strong garlic-like odor, and its rhizome serves as a reservoir of organic compounds and water. The stem height can reach up to 2.5 m. Experimental studies were conducted in the territories of “Qiziriq” and “Boysun” state forestry enterprises in Surkhandarya region, where biogels were applied to F. tadshikorum plants and plantations were established. The average annual temperature in these regions is approximately 16–18°C. During the coldest months (January–February), minimum temperatures may fall to −13°C, while maximum values range from +3 to +6°C. In summer (July), maximum temperatures reach +33–35°C, occasionally exceeding +40°C. In April, average temperatures are +16°C (day) and +7°C (night). Rainfall is scarce in August, with average temperatures of +28/+16°C. Annual precipitation is about 400–460 mm, with the wettest period occurring from February to April (77–86 mm per month), while summer precipitation is minimal (~5 mm). Snowfall occurs from November to March, with the highest levels recorded in January (~54 mm).The propagation of F. tadshikorum using biogel technology was studied in these conditions, with emphasis on its biomorphological characteristics. For determining morphological traits and phenological phases, the methods of I.N. Beydeman (1974) and G.E. Shulz (1966) were applied.
3. Results and Discussion
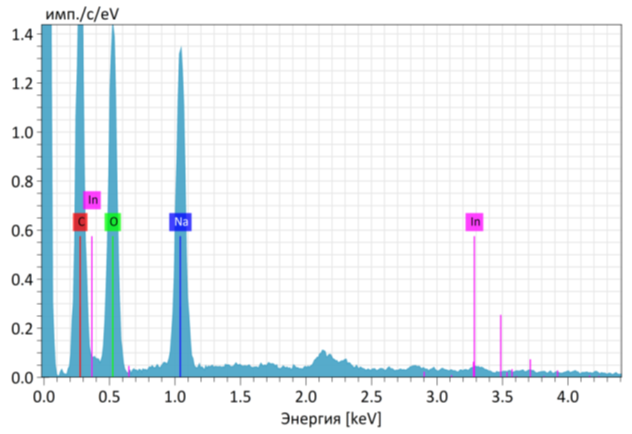
- Using carboxymethyl cellulose (CMC) derived from cellulose waste, an environmentally friendly hydrogel was synthesized. This hydrogel is biodegradable, non-polluting to soil, and exhibits a high water-retention capacity. For synthesis, 6 g of CMC and 2 g of polyvinyl alcohol (PVA) were dissolved in 100 ml of distilled water. The mixture was stirred on a magnetic stirrer at 90°C until complete dissolution was achieved. After stirring, the solution was placed in a −40°C freezer for 24 hours. Upon removal, the frozen samples were thawed, and the freeze–thaw cycle was repeated several times to enhance gel formation. After the final thawing, the samples were incubated in a thermostat at 20°C for 24 hours.Following incubation, the physicochemical properties of the hydrogel were investigated. The water absorption capacity was determined using the gravimetric method. It was found that 1 g of hydrogel could absorb approximately 220–280 ml of water under laboratory conditions, demonstrating its strong hydrophilic nature.In the subsequent stage, the morphology of the hydrogel was studied using Scanning Electron Microscopy (SEM). The analysis revealed the porous architecture of the hydrogel, while elemental composition was determined through energy-dispersive X-ray (EDX) analysis (Figure 1). These structural and compositional features confirmed the suitability of the synthesized hydrogel for biological immobilization and agricultural applications.
 | Figure 1. SEM image of the hydrogel synthesized from carboxymethyl cellulose (CMC) at 20 kV, ×2.5k magnification, within a 30 μm scale range |
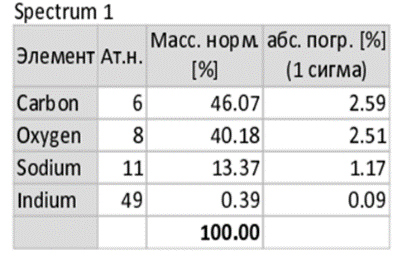 | Table 1. Quantitative elemental composition of the hydrogel |
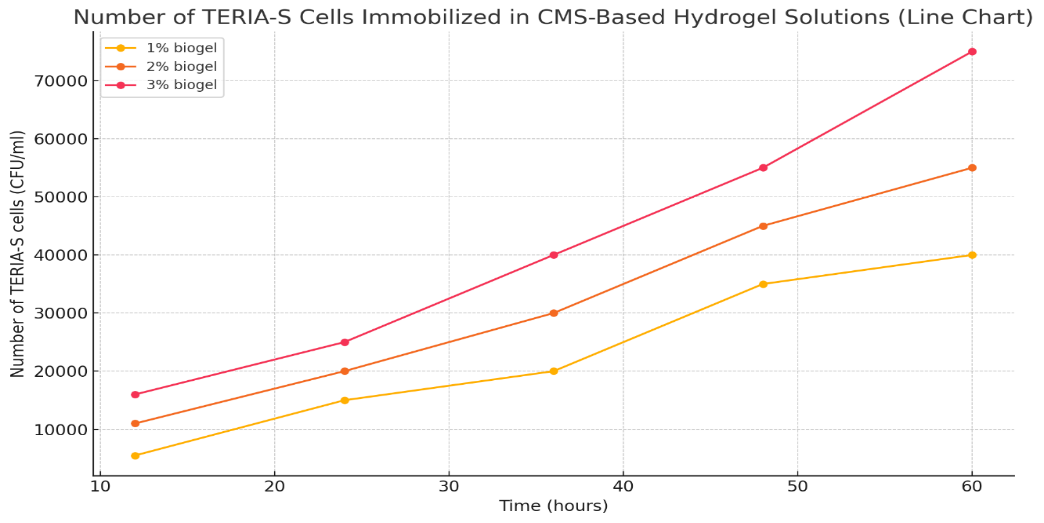 | Table 2. Number of Teria-s cells immobilized in the hydrogel at different incubation times (12–60 h), (CFU/mL) |
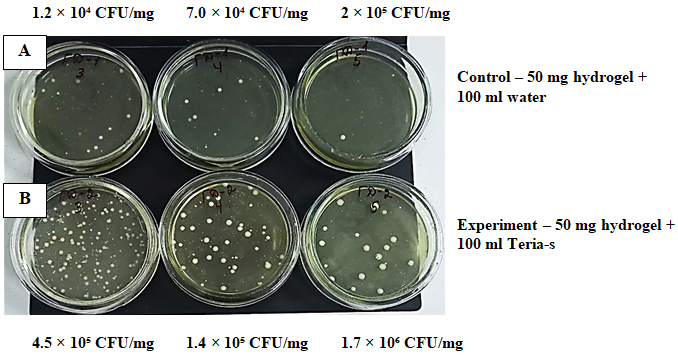 | Figure 3. Viability of TERIA-S bacteria: A – bacteria in the biogel composition; B – sample placed in sterile distilled water as a control |
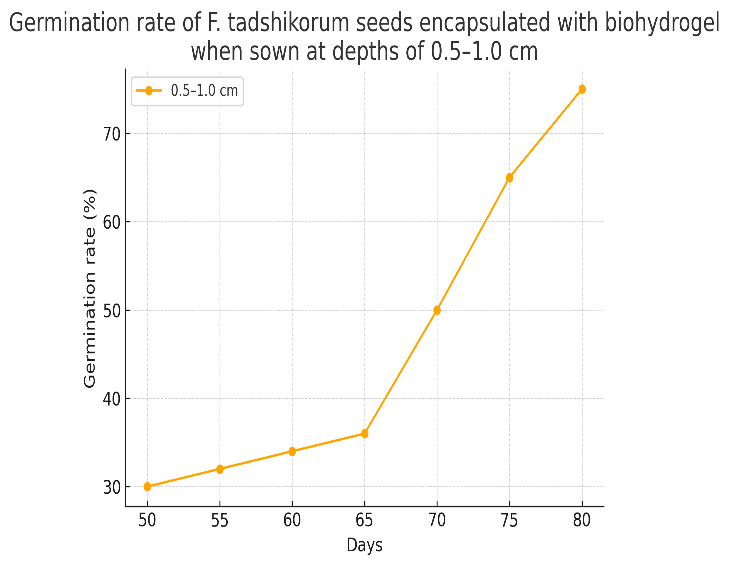 | Table 3. Germination rate of F. tadshikorum seeds encapsulated with biohydrogel when sown at depths of 0.5–1 cm |
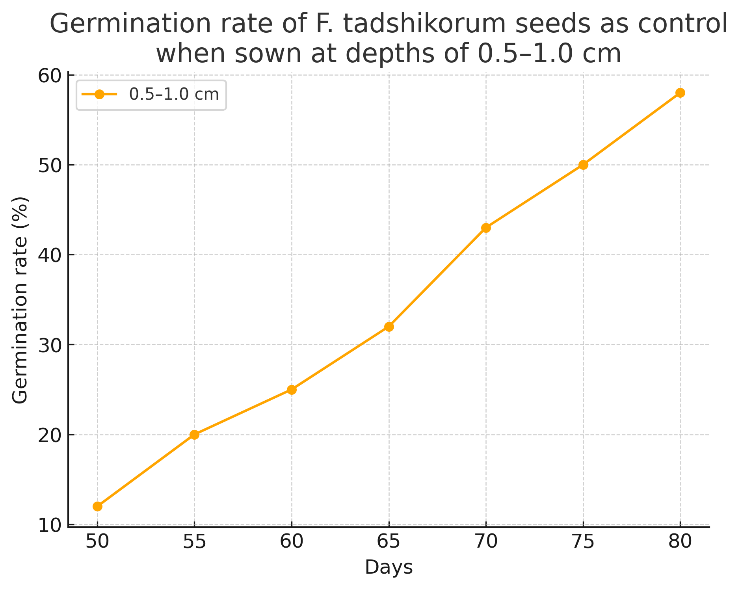 | Table 4. Germination rate of F. tadshikorum seeds as control when sown at depths of 0.5–1 cm |
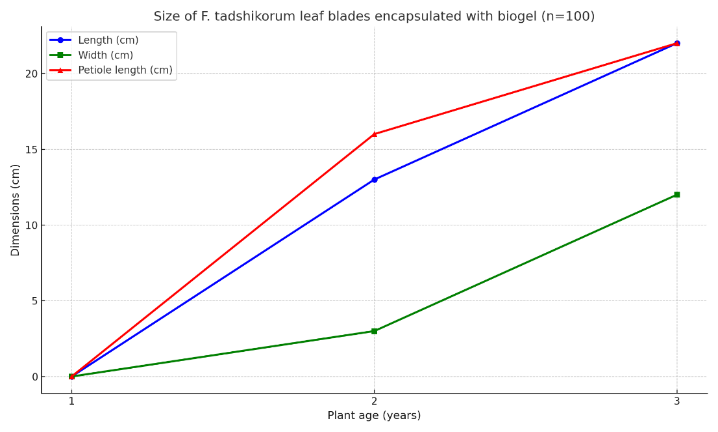 | Table 5. Leaf size of F. tadshikorum when seeds were sown encapsulated with the biogel (n=100) |
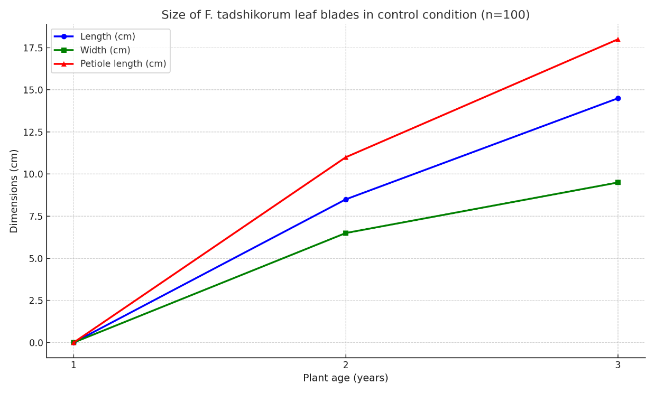 | Table 6. Leaf size of F. tadshikorum when seeds were sown as control (n=100) |
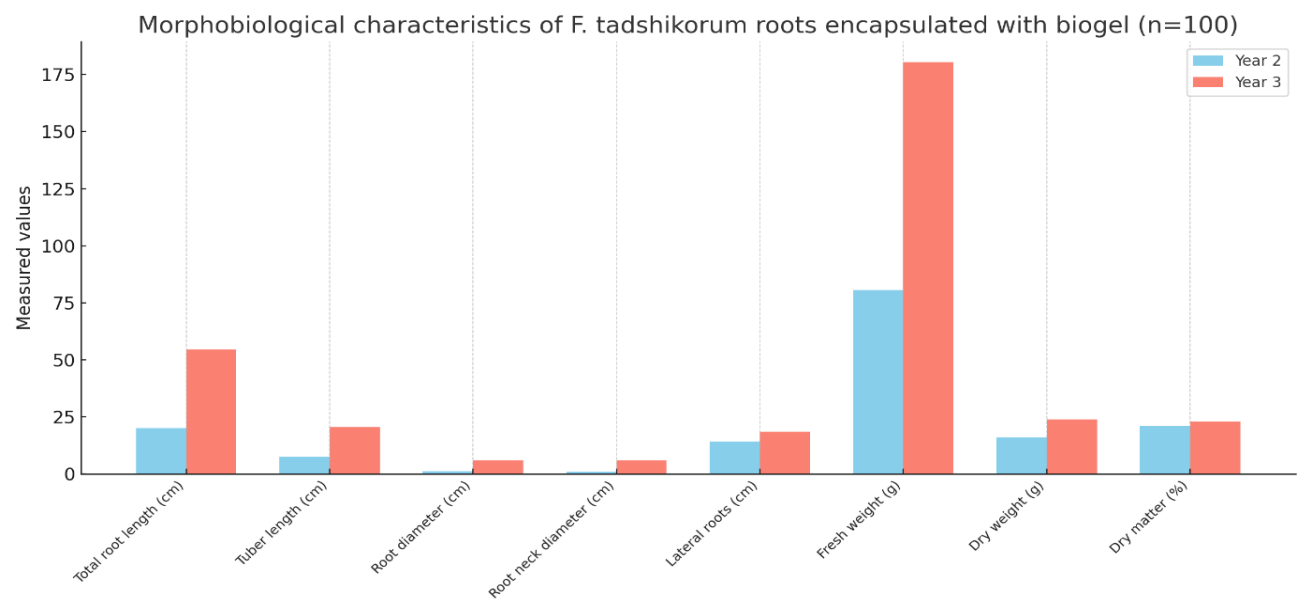 | Table 7. Morphobiological classification of F. tadshikorum roots when seeds were sown encapsulated with the biogel (n=100) |
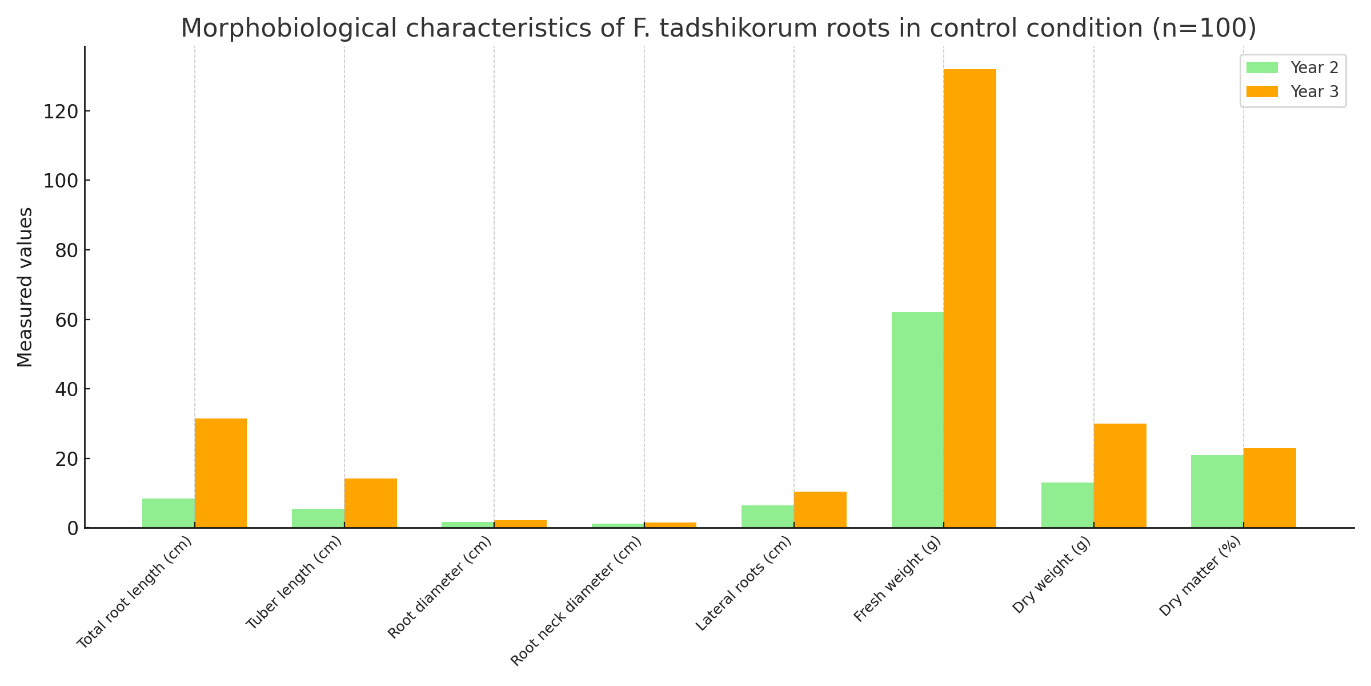 | Table 8. Morphobiological classification of F. tadshikorum roots when seeds were sown as control (n=100) |
4. Conclusions
- Within the framework of this study, a hydrogel was synthesized on the basis of natural polymers such as carboxymethyl starch (CMS), and TERIA-S bacterial cells were immobilized into its structure, resulting in the creation of an environmentally safe and biologically active biogel. The main advantages of the synthesized hydrogel are related to its biodegradability in soil, hydrophilic properties, the ability to preserve bacterial viability, and its capacity to retain essential macro- and microelements required for plants. Due to these properties, it demonstrated high agrobiotechnological efficiency not only under laboratory but also under field conditions. The immobilization of TERIA-S bacteria within the biogel further enhanced its biological activity, ensuring the long-term viability of microorganisms within the hydrogel and guaranteeing their beneficial activity in the rhizosphere. Such a system stimulates plant nutrition, increases resistance to stress conditions, and accelerates overall growth.Field experiments revealed that when Ferula tadshikorum seeds were sown at a depth of 0.5–1.0 cm in an encapsulated biogel form, their germination rate increased by 30–40% compared to the control group. The biogel not only created an optimal microclimate around the plants and retained water for an extended period but also supplied essential elements. Particularly under conditions of water scarcity, salinity, and sandy soils, the water retention and nutrient carrier functions of the biogel proved to be crucial. Indicators of the root system of F. tadshikorum (root length, diameter, tuber formation, weight, and dry matter content) were significantly higher in the biogel-treated samples compared to the control group. Furthermore, leaf size and vegetative development stages were accelerated under the influence of the biogel. These results indicate that this approach is effective not only under experimental conditions but also in real agro-technical environments.The practical significance of this experiment lies in the fact that medicinal plants such as F. tadshikorum can be successfully cultivated even in water-deficient and resource-limited environments. Field trials conducted in rainfed lands of the Surkhandarya region confirmed that the use of biologically active biogel significantly increases seed germination, agrobiological stability, and productivity of medicinal plants. As a result, large-scale plantations can be established, the raw material base can be stabilized, and the quality of bioactive compounds can be improved.In conclusion, the synthesized CMS-based biogel represents an innovative approach in agricultural technology, playing an important role in developing ecologically safe, economically efficient, and scientifically substantiated methods for plant cultivation. This, in turn, is a promising outcome that can be widely applied in the fields of pharmaceuticals, agrochemistry, and environmental protection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML