-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(9): 204-211
doi:10.5923/j.ijge.20251309.07
Received: Aug. 28, 2025; Accepted: Sep. 16, 2025; Published: Sep. 29, 2025

Scientific Basis for the Selection and Adaptation of Soybean (Glycine Max L.) Varieties Based on Morphophysiological Traits
Rakhimova Kholiskhon Maksudovna1, Bobojonova Khulkar Madrahimovna2, Bobojonova Gulnoza Farhodovna3, Jumabayeva Dilnoza Davronbekovna3, Dilnoza Kakhramonovna Kamiljonova4
1PhD in Biological Sciences, Associate Professor, Department of Biology, Urgench State University, Urgench, Uzbekistan
2Lecturer, Department of Biology, Urgench State University, Urgench, Uzbekistan
3Master’s Student, Department of Biology, Urgench State University, Urgench, Uzbekistan
4Lecturer at Ranch University, Urgench, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Soybean (Glycine max L.) is one of the most important legume crops in the world, valued for its high protein content, oil yield, and significant role in sustainable agriculture. In the northern regions of Uzbekistan, where climatic conditions are characterized by high temperatures, soil salinity, and limited water resources, the selection of soybean varieties based on morphophysiological traits is of great practical and scientific importance. This review highlights the key morphophysiological indicators, including plant height, leaf area, pod formation, seed weight, photosynthetic activity, chlorophyll content, transpiration efficiency, and nitrogen fixation potential. These traits serve as important criteria for evaluating varietal performance and adaptability under abiotic stress factors such as drought and salinity. The study emphasizes the necessity of identifying varieties with high ecological adaptability, stable yield potential, and improved stress tolerance. Comparative analysis of local and introduced soybean varieties demonstrates that morphophysiological markers can be effectively used for varietal selection, breeding programs, and the development of agro-biological recommendations suitable for the northern regions of Uzbekistan. The findings provide a scientific basis for the adaptation of soybean cultivation to regional conditions, contributing to sustainable agriculture, food security, and enhanced resilience of agroecosystems.
Keywords: Soybean (Glycine max L.), Varieties, Morphophysiological traits, Selection, Adaptation, Drought tolerance, Salinity, Sustainable agriculture, Uzbekistan
Cite this paper: Rakhimova Kholiskhon Maksudovna, Bobojonova Khulkar Madrahimovna, Bobojonova Gulnoza Farhodovna, Jumabayeva Dilnoza Davronbekovna, Dilnoza Kakhramonovna Kamiljonova, Scientific Basis for the Selection and Adaptation of Soybean (Glycine Max L.) Varieties Based on Morphophysiological Traits, International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 204-211. doi: 10.5923/j.ijge.20251309.07.
Article Outline
1. Introduction
- Soybean (Glycine max L.) is one of the most economically important legume crops worldwide, widely cultivated for its high protein and oil content as well as its ability to improve soil fertility through biological nitrogen fixation. In recent decades, soybean has become increasingly significant for food security, livestock feed, and industrial applications [2,4,5].In Uzbekistan, the expansion of soybean cultivation is of strategic importance, particularly in the northern regions where environmental conditions are characterized by aridity, high soil salinity, and temperature fluctuations. These abiotic stresses significantly limit the productivity and stability of soybean crops, making the selection of stress-tolerant and high-yielding varieties a priority.Morphophysiological traits such as plant height, leaf area, number of pods per plant, seed weight, chlorophyll content, photosynthetic efficiency, transpiration rate, and nitrogen fixation capacity play a crucial role in determining the adaptability and yield potential of soybean varieties. Evaluating these traits provides a reliable scientific basis for identifying the most suitable varieties under challenging agro-ecological conditions [1,3].Given the growing demand for soybean and the need to increase its cultivation in marginal environments, the study of morphophysiological indicators is essential for developing effective selection criteria and adaptive cultivation strategies. This approach contributes to sustainable agriculture, enhances crop resilience, and supports regional food security [4,7,25,26].Literature review. Morphophysiological traits connect plant form with function under stress and therefore serve as fast, quantitative indicators of adaptation before final yield is known. Core trait groups repeatedly associated with resilience include: (i) leaf gas exchange (photosynthetic rate, stomatal conductance, transpiration) and derived water-use efficiency; (ii) chlorophyll status and photochemistry (SPAD, Fv/Fm); (iii) canopy architecture (specific leaf area, LAI), (iv) root system architecture and vigor; (v) symbiotic nitrogen fixation (nodule number/biomass, effectiveness); (vi) osmotic adjustment (proline, glycine betaine); (vii) ion homeostasis under salinity (K⁺/Na⁺); and (viii) antioxidant capacity (SOD, CAT, POD). Recent controlled and field studies confirm that these traits discriminate tolerant vs. sensitive genotypes across heat, salinity, drought, and waterlogging, and they can be integrated into selection indices for specific environments [8,10,13,15,20]. Temperature is a first-order driver of soybean development, seed set, and composition. Across temperature gradients, cultivar-specific optima emerge for pods plant⁻¹, seed number, and seed yield; when parental plants experience high heat during pod filling, their offspring show reduced germination-evidence of transgenerational penalties that breeding and seed production systems must consider. Complementary work indicates that exposure to ~38/30°C during seed development depresses yield and alters oil quality (elevated monounsaturated fatty acids), with elevated CO₂ unable to offset the heat penalty. Together, these studies justify screening candidate varieties under realistic thermal regimes and including reproductive-stage measurements (e.g., pollen viability, canopy temperature) in selection panels [21,23,25]. Under concurrent drought and salinity, tolerant genotypes maintain photosynthesis and chlorophyll, stabilize membranes, accumulate osmolytes (proline, glycine betaine), and sustain ROS-scavenging enzymes (SOD, CAT, POD). They also preserve higher relative water content and regulate Na⁺/K⁺ to protect photosystems and growth. A recent multi-trait study that combined physiology, osmolytes, water relations, and expression of stress-responsive genes (e.g., GmNHX1, GmAKT1, GmDREB1) provides a practical template for breeding nurseries and adaptation trials. Likewise, independent work shows graded NaCl treatments reduce plant height, leaf number, root/shoot growth, and biomass-traits that, together with K⁺/Na⁺ ratios, can form reliable early-stage screening metrics. In irrigated lowlands and heavy soils, transient waterlogging impairs roots and whole-plant growth. A 2024 comparative study of two introgression lines (waterlogging-tolerant vs. sensitive) demonstrated that tolerance is associated with better maintenance of root length/biomass, chlorophyll and photosynthesis, and coordinated biochemical and transcriptomic responses. This supports using combined root and shoot traits-along with chlorophyll/photochemistry and redox indicators-as a screening package for waterlogged environments [1,6,8,9]. Physiological trait differences under salinity have a genetic basis that can be leveraged in selection. Recent reviews and genome-wide association studies (GWAS) map salt-tolerance components (e.g., ion transport, membrane integrity) to specific loci, enabling marker-assisted or genomic selection to complement physiological screening and accelerate gain. A 2024 GWAS across diverse wild and cultivated accessions identified novel loci for germination-stage salt tolerance (germination rate, energy, index), facilitating early-generation screening that links genetic markers to physiological performance [11,13,15]. Symbiotic nitrogen fixation and local compatibility. Uzbek teams isolated numerous Bradyrhizobium strains from nodules of local varieties (e.g., Dostlik, Parvoz, Nafis). They documented region-specific nodulation responses and highlighted that some foreign inoculants fail to nodulate local varieties, whereas locally adapted strains form abundant, effective nodules. Because leaf N status drives chlorophyll and photosynthesis, inoculant–host matching is a prerequisite for fair physiological comparisons among varieties in Uzbekistan. Raximova Kh.M., Yormatova, and others studied biometric indicators in soybean (growth rate, stem height, number of nodules) and determined the level of adaptability of different varieties to local conditions.Shakirov Z.S. and colleagues (2023) isolated nodule bacteria from Uzbek soybean varieties and proved that local strains are more effective than foreign inoculants. This demonstrates that the efficiency of symbiosis directly affects physiological processes such as photosynthesis and yield.Kholikova M. (2024) and other researchers reported that some foreign varieties (Sparta, Oyjamol) also showed high productivity under the conditions of Uzbekistan, but emphasized the need for deeper analysis of their physiological basis.
2. Research Methodology
- Experimental site and conditions. The research program spanned 2022–2025, with field trials conducted at the experimental fields of Urgench State University and on selected farmers’ fields in the Khorezm region of Uzbekistan. The trial soils are classified as meadow-alluvial, medium-textured, with moderate salinity.The climate of the region is sharply continental, characterized by hot summers (28–35°C), low annual rainfall, and strong dependence on irrigation for crop cultivation.Plant material. A total of 13 soybean varieties were studied: Nafis (control variety), Nofer, Evrika-357, Fortuna,Vilana, Selekta-302, Parvoz, BK-34, Genetika-1, Extiyoj, Xotira, BK-61, Sochilmas. These varieties were tested both as main crops and as repeated crops after wheat. Experimental design and treatments. The experiment was laid out in a Randomized Complete Block Design (RCBD) with three replications. The following fertilizer treatments were applied:1. N60 P₂O₅60 K₂O602. N40 P₂O₅80 K₂O603. N80 P₂O₅60 K₂O904. Control (without fertilizers)Soybean was sown in rows with a spacing of 60 cm between rows and 5 cm between plants within the row. The seed rate was adjusted according to agro-technical requirements. Morphological observations. Throughout the growing season, the following morphological traits were recorded: Plant height (cm), Number of branches per plant, Number of leaves and leaf area index (LAI), Duration of flowering and maturity stages, Root length and biomass.Physiological observations. Physiological parameters were measured at the vegetative and reproductive stages: Chlorophyll content (SPAD index), Photosynthetic rate, transpiration rate, Relative water content (RWC). Yield and yield-attributing traits. At harvest, the following yield traits were measured: Number of pods per plant, Number of seeds per pod, Thousand-seed weight (g), Seed yield per hectare (t/ha).Statistical analysis. Data were subjected to analysis of variance (ANOVA) using RCBD design. Mean comparisons were carried out with the Least Significant Difference (LSD) test at p ≤ 0.05. Correlation and regression analyses were performed to determine relationships between morphophysiological traits and yield. Principal component analysis (PCA) was used to identify the most discriminating traits for varietal selection and adaptation.
3. Analysis and Results
- The statistical analysis of the experimental results showed that the yield of the 13 studied soybean varieties differed significantly depending on the fertilizer application rates (p<0.05). The differences in plant growth and development were mainly explained by their genotypic characteristics and adaptability to agro-technical practices.The leading varieties in terms of yield were identified as Nofer, Parvoz, Evrika-357, Fortuna, and Genetika-1. These varieties developed a strong root system during the growing season, had higher green leaf mass, and maintained prolonged photosynthetic activity, which contributed to higher seed formation. Particularly, their ability to efficiently assimilate nutrients during the seed-filling period played a decisive role in increasing productivity.Under the conditions of Khorezm, in non-inoculated soils (i.e., without the artificial addition of rhizobacteria), the most optimal fertilizer regime was N60P60K60. This treatment ensured a balanced development of vegetative and generative organs, thereby enabling higher yields. Such results are of practical importance, especially for soils where biological nitrogen fixation processes are weak.In the control treatment (without fertilizers), yields were low across all varieties. This confirmed the necessity of mineral fertilizers for effective soybean growth and development. In unfertilized plots, leaf chlorosis, shorter vegetation periods, and incomplete seed formation were observed.In addition, some varieties (Nafis, Extiyoj, Xotira, and Sochilmas) demonstrated stability but failed to achieve high yields. This was related to their genetic characteristics, sensitivity to ecological stress factors, and limited ability to utilize soil nutrients. Therefore, while these varieties may serve as valuable genetic resources, their recommendation for commercial cultivation remains limited.In general, the results confirmed that soybean varieties respond differently to agro-technical practices. The N60P60K60 regime was found to be the most optimal under non-inoculated conditions, as it provided an adequate balance of nitrogen, phosphorus, and potassium for plant growth and development. This not only supported the activity of the photosynthetic apparatus but also ensured a continuous nutrient supply during seed formation and filling.
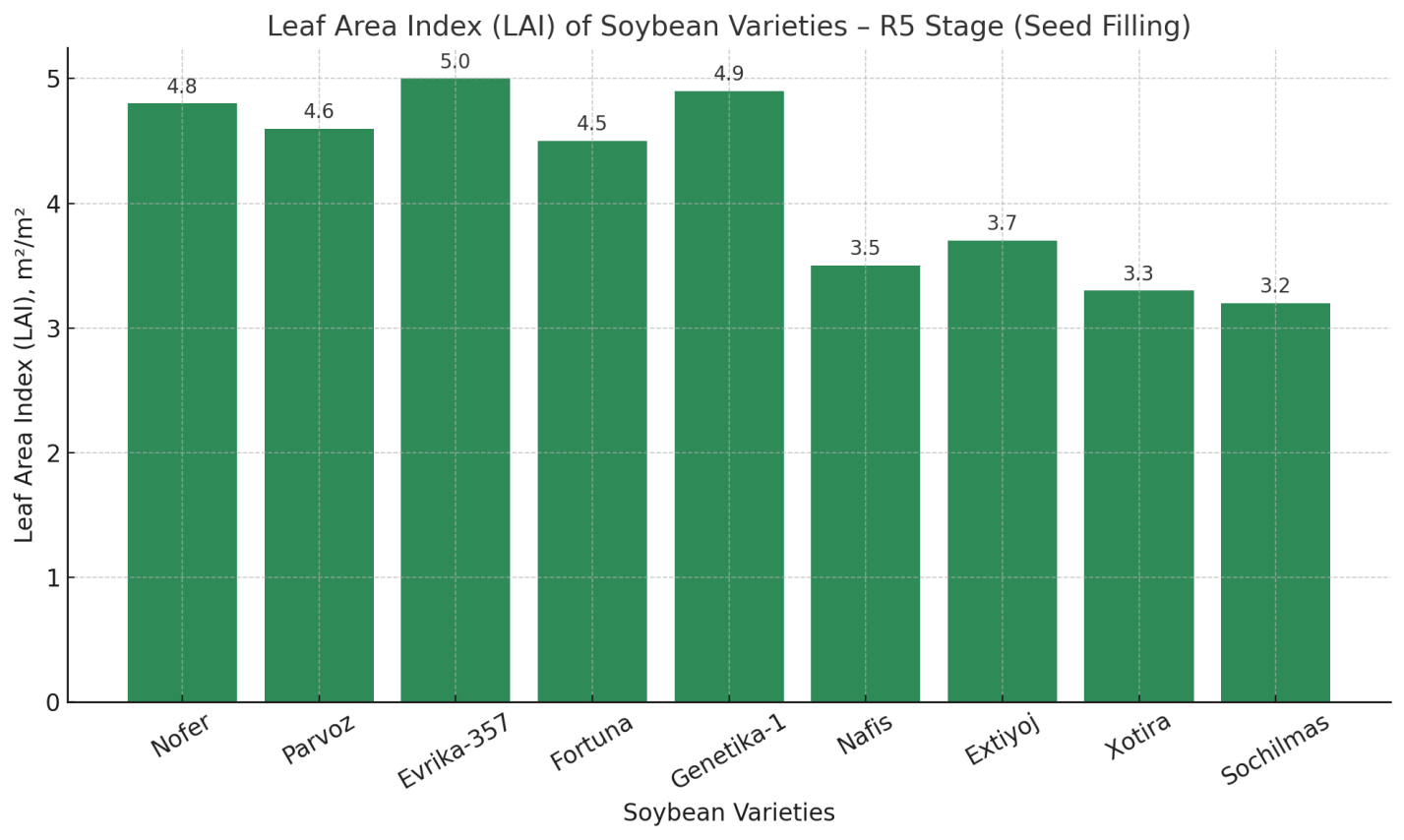 | Figure 1. Leaf Area Index (LAI) of soybean varieties – R5 stage (Seed filling) |
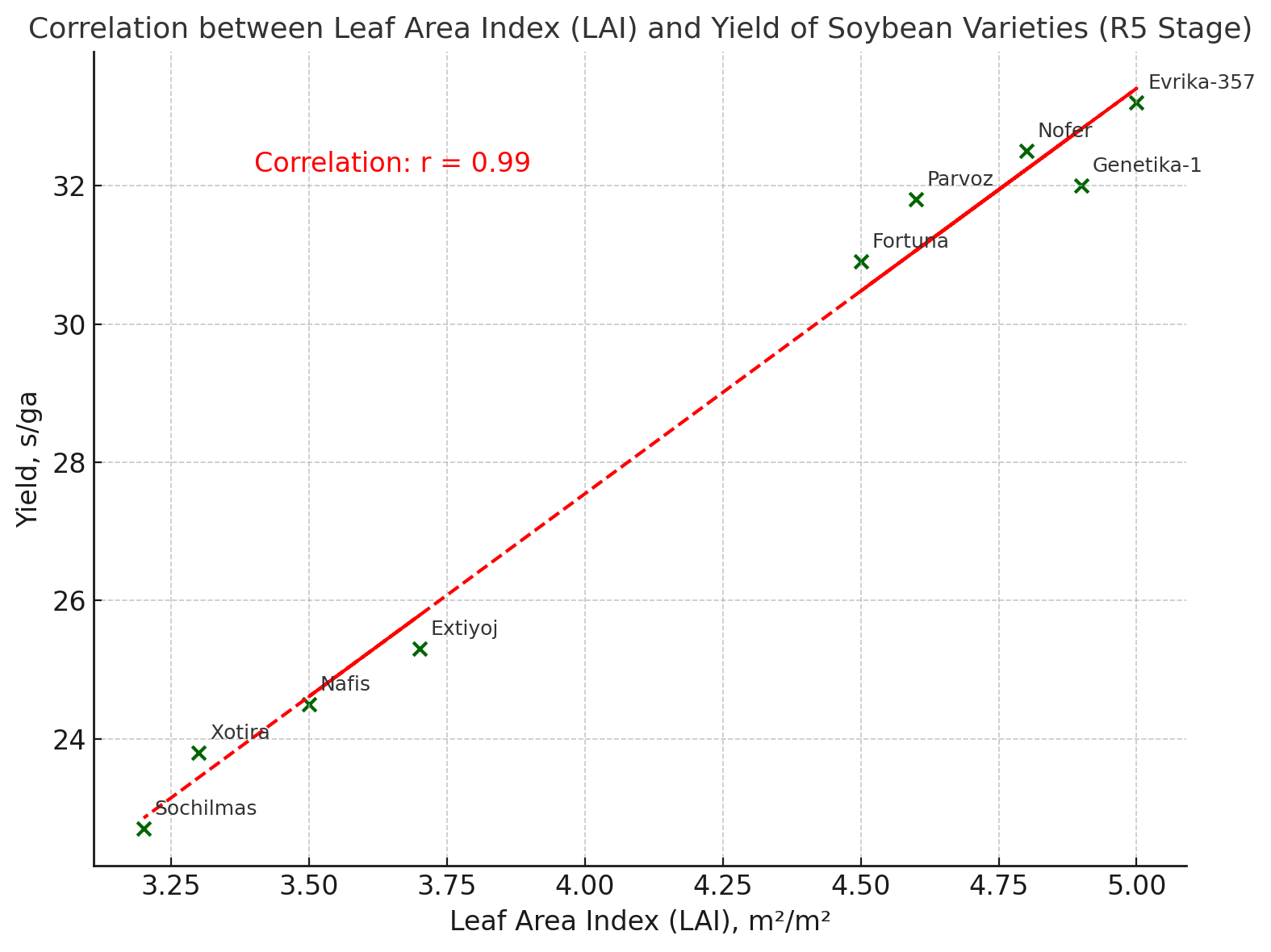 | Figure 2. Correlation between (LAI) and yield of soybean varieties (R5 Stage) |
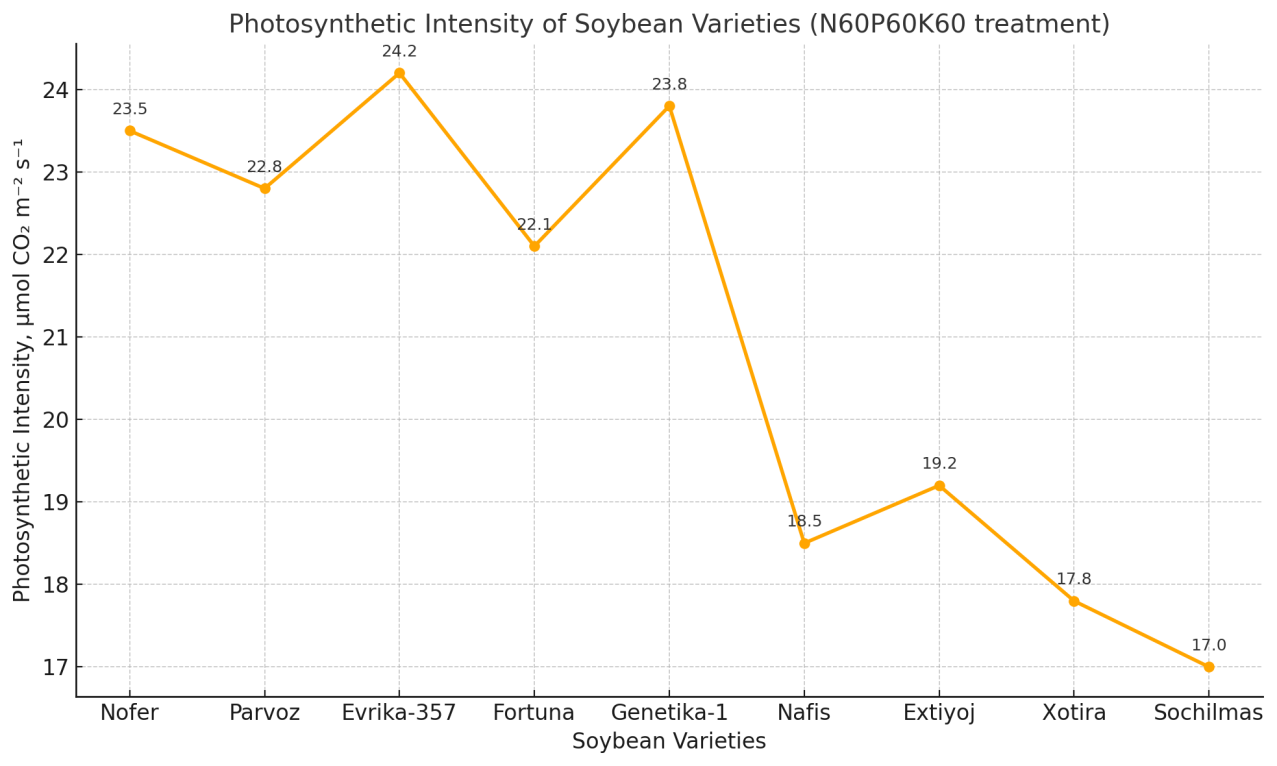 | Figure 3. Photosynthetic intensity of soybean varieties (N60P60K60 treatment) |
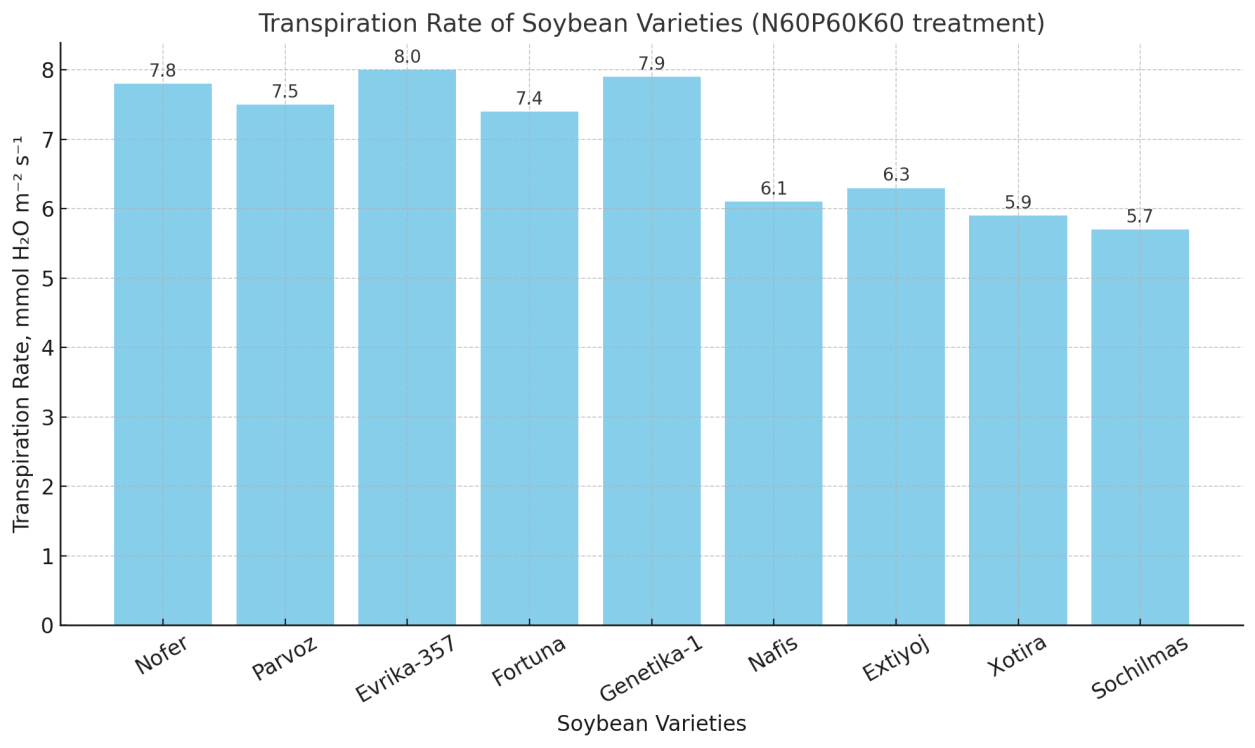 | Figure 4. Transpiration rate of soybean varieties (N60P60K60 treatment) |
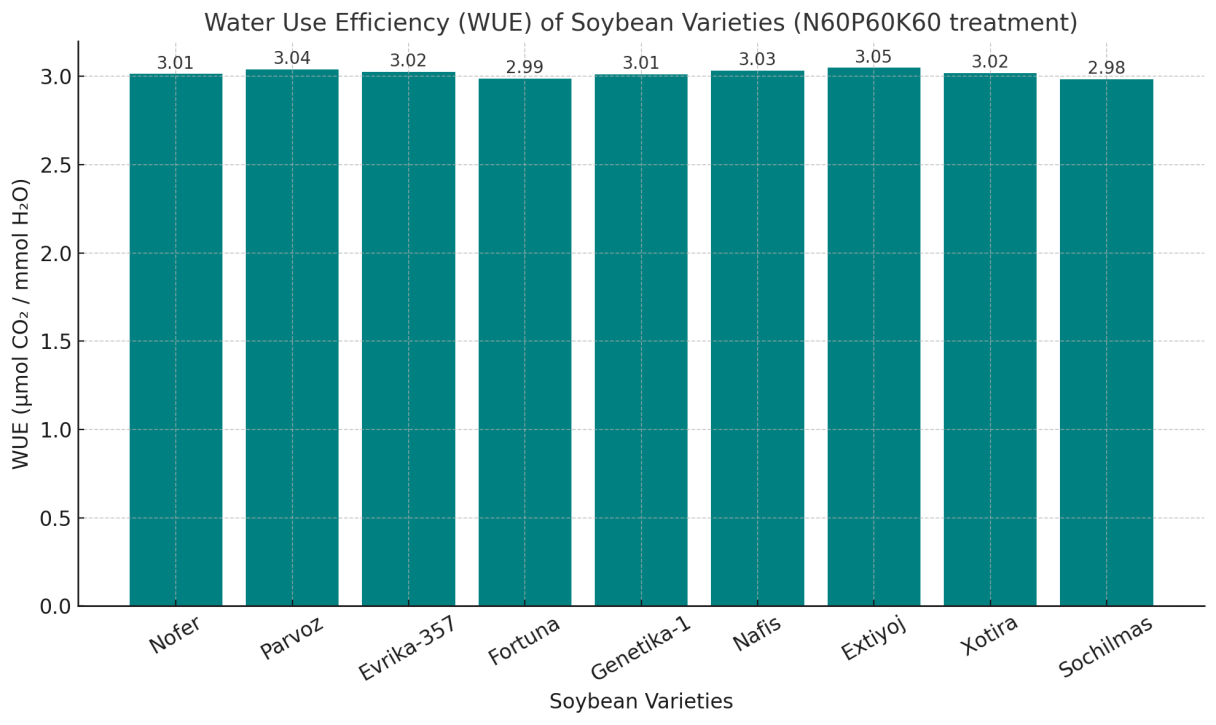 | Figure 5. Water use efficiency (WUE) of soybean varieties (N60P60K60 treatment) |
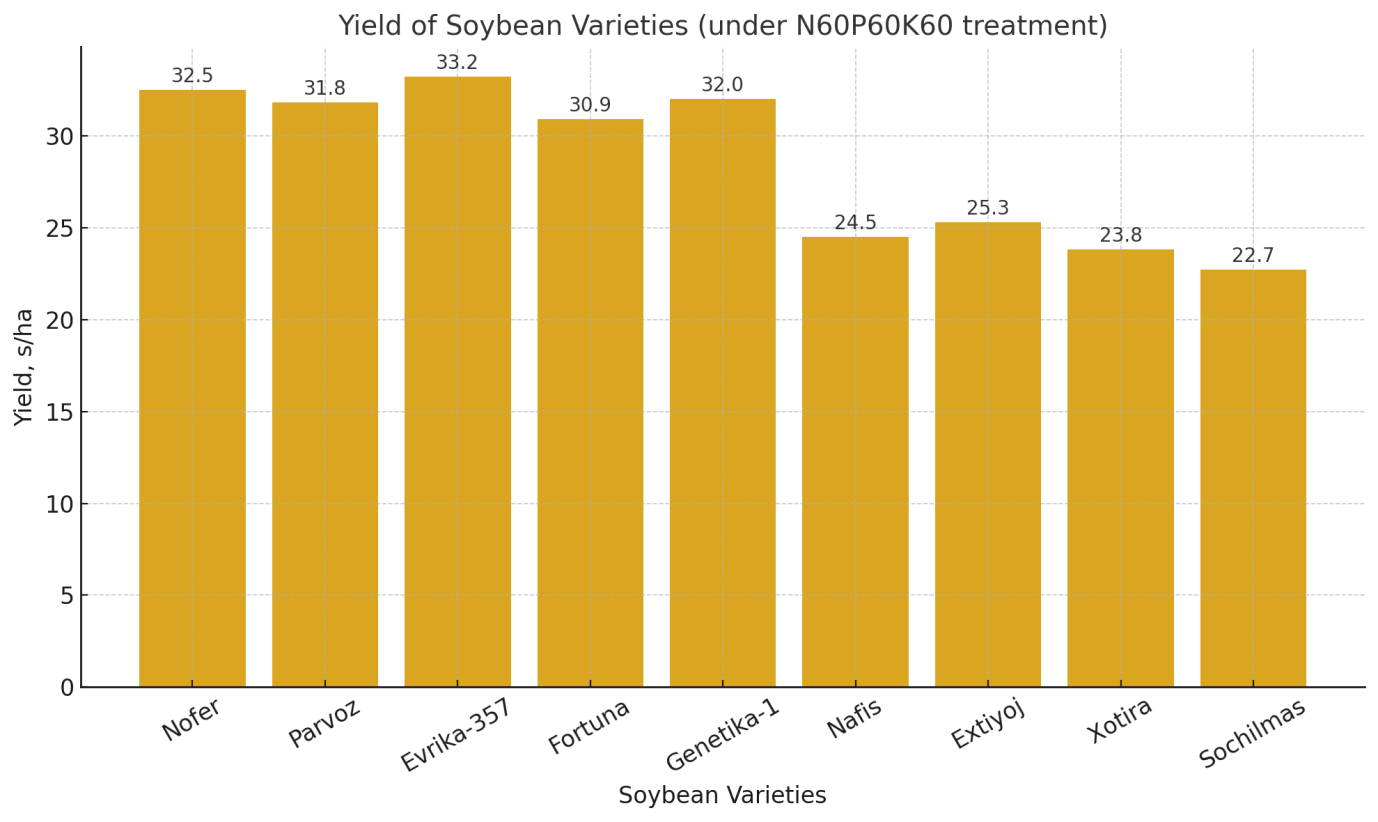 | Figure 6. Yield of soybean varieties (under N60P60K60 treatment) |
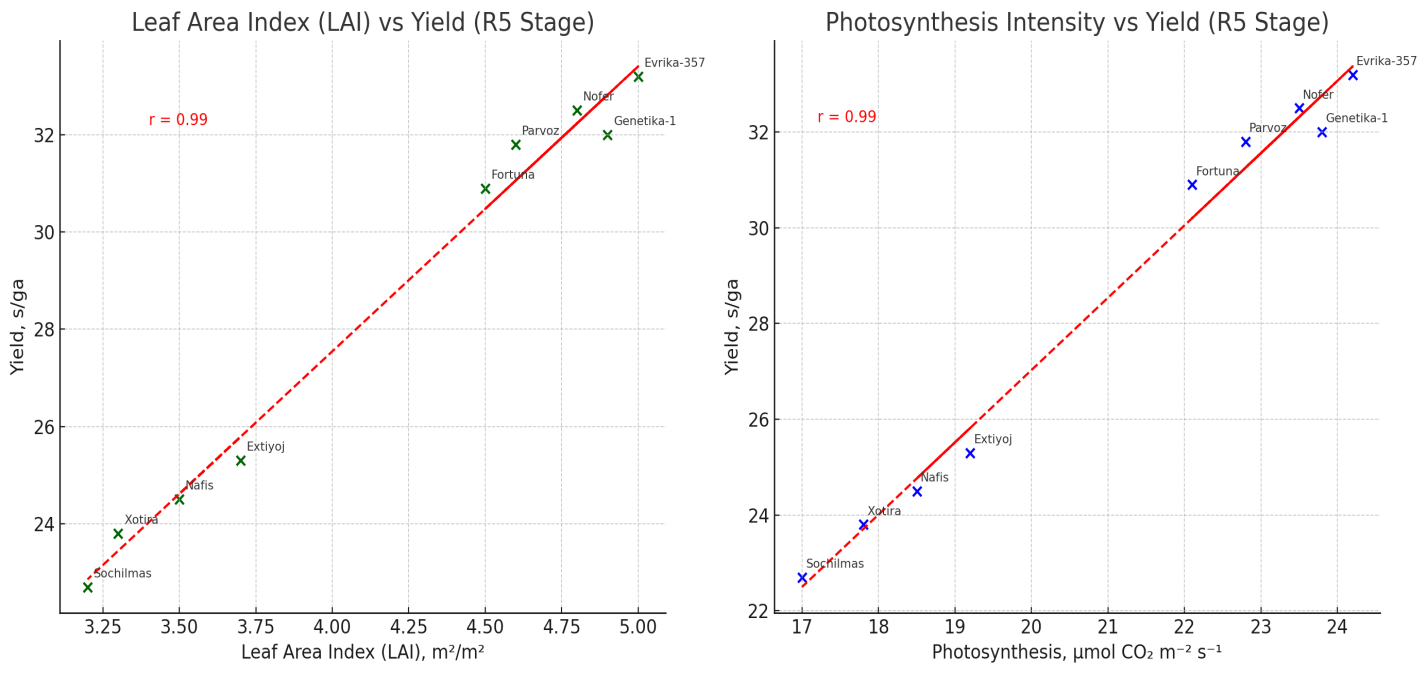 | Figure 7. Combined figure with two key relationships at the R5 stage (seed filling) |
4. Conclusions
- Soybean varieties showed significant variation in yield and physiological traits under the N60P60K60 fertilization regime in Khorezm conditions. Yield formation was strongly correlated with photosynthetic intensity and leaf area index (r ≈ 0.98–0.99). High-yielding varieties such as Evrika-357, Nofer, and Genetika-1 combined superior photosynthetic activity, optimal LAI, and higher productivity (30–33 s/ha), while Nafis, Extiyoj, Xotira, and Sochilmas demonstrated weaker performance. Thus, physiological efficiency is a decisive factor in selecting soybean varieties for regional adaptation.
5. Recommendations
- 1. Fertilization practice. The N60P60K60 regime should be considered the optimal fertilization strategy for soybean cultivation in Khorezm under non-inoculated conditions.2. Varietal selection. Evrika-357, Nofer, and Genetika-1 can be recommended as the most productive and adaptive varieties for large-scale cultivation.3. Water management. Monitoring transpiration and enhancing water use efficiency (WUE) is critical for sustainable production, especially under water-limited conditions.4. Future research. Combining mineral fertilization with rhizobial inoculation should be investigated further to improve nutrient assimilation, biological nitrogen fixation, and overall yield stability.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML