Sabokhat Tashpulatova Akbarovna
PhD., Department of Ecology, National University of Uzbekistan, Tashkent, Uzbekistan
Correspondence to: Sabokhat Tashpulatova Akbarovna, PhD., Department of Ecology, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Samples were taken from 70 spring water resources distributed in the Gallaorol district of Jizzakh region in Uzbekistan and their drinking and healing properties were analyzed. To analyze the water samples, gravimetric, photometric, complexometric, argenometric, spectrophotometric, titrometric, atomic adsorption and other physicochemical methods were used to determine the pH, SiO2, dry residue, Ca2+, Mg2+, Fe (total), CO32-, HCO3, SO42-, Cl-, NO2-, NO3-, total hardness, heavy metals, and organic matter content. According to the results of the analysis, 61 of the 70 spring water resources studied were suitable for drinking (87%), and 9 (13%) were unsuitable. It was determined that all of the springs suitable for drinking had high organoleptic quality water, but were not mineral water.
Keywords:
Spring waters, Drinking water, Healing properties, Healing mineral water
Cite this paper: Sabokhat Tashpulatova Akbarovna, Identification and Evaluation of Drinking and Healing Properties of Springs (Gallaorol District, Uzbekistan), International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 193-203. doi: 10.5923/j.ijge.20251309.06.
1. Introduction
Springs are natural and are formed as a result of processes between underground water and rock [1-4]. Springs are divided into 2 types: hot and quiet springs. Hot springs - emerge from the ground under the influence of gas, hydrostatic pressure, and steam. Such springs are found in abundance in the Fergana Valley (Shorsuv, Uzbekistan). Quiet springs - arise when springs emerge from the ground as a result of the blockage or opening of impermeable rocks in front of aquifers. Such springs are common in the Fergana Valley, Zarafshan, and Kashkadarya regions are widely used by the population [5-12]. Springs are common in the foothills of the Fergana, Zarafshan, Kashkadarya, Nurota, Jizzakh, and Tashkent oases of our country. In these areas, the water of some springs reaches 10-15 l per second [13]. Water quality depends on various physical and chemical parameters, such as: pH, total dissolved salts, CO2, total hardness, heavy metals, anions and cations, their content and composition [14-17].Springs in the Gallaorol district of Jizzakh region serve as the only source of drinking water for most rural residents, and for thousands of years, springs have been used for irrigation of household and agricultural purposes, as a source of healing mineral water, for recreation, swimming and diving, and as a tourist destination [18].Factors such as total hardness, pH, and heavy metal content of spring water affect water quality indicators, and their content, in turn, affects human health. In particular, the total hardness of water depends on the calcium and magnesium carbonate compounds in the water, and an excessive amount of these compounds causes negative consequences such as heart defects and salt accumulation in the kidneys [19-21].Qualitative and quantitative monitoring and analysis of spring waters is the first condition for being aware of the state and level of pollution of groundwater. The human factor remains one of the main causes of pollution of groundwater, especially spring water resources [22]. Spring waters also contain factors that have a positive effect on the human body and health, namely minerals and beneficial substances (CO2 free (dissolved), H2S generally, Fe (Fe2++Fe3+), Br, J, H2SiO3+HSiO3-, H3BO3, organic matter, Rn, reaction of water, temperature) [23]. Accordingly, 241 samples were collected from 70 spring water resources in the study area and the following goals were set:a. Determination of the physical properties of spring waters.b. Analysis of the chemical composition of spring waters.c. Comparison and evaluation of the results obtained with the state standard requirements for drinking and mineral water.
2. Materials and Methods
2.1. Study Area
Gallaorol district is located in the Jizzakh region at 40′01′17 north latitude and 67′35′51 east longitude, that is, in terms of territorial division, it is located in the western part of the region [18]. Gallaorol district is bordered by Nurata in the north, Turkestan in the south, Kuytash in the northeast, Malguzar in the southeast, and Gubdintog ranges in the west [20-23]. The climate of the district is unique, that is, precipitation is seasonal, with slightly more precipitation in winter and early spring. The climate is sharply continental, with rapid temperature changes even during the day, with many sunny days, little precipitation, and very dry weather [20]. The Gallaral district has unique climatic characteristics that differ from the climate of other regions of our country, namely, it does not have thick snow cover in winter and does not rain for long periods [21].
2.2. Research Design
Water samples were tested in the Navoi Mining and Metallurgical Water Laboratory, the Jizzakh Regional Sanitary Epidemiology Center Water Laboratory, and the State Unitary Enterprise of Hydrogeology during 2020-2023. Using the GPSMAP 64sx device, the topographic indicators (geographic coordinates) of each spring water resource were determined.
2.3. Sampling Procedure
A total of 241 water samples from 70 spring water resources were collected in pre-sterilized, dried, and prepared polyethylene containers and sent to the laboratory for chemical analysis.
2.4. Data Collection and Analysis
All spring water samples were subjected to physical and chemical analysis. The data were analyzed using Microsoft Excel computer software.
2.4.1. Physical Quality Analysis Methods
The physical examinations focused mainly on water properties and pH. The table pH meter was used to measure the pH level of the Gallaorol district spring water. The electrode was immersed in the sample.
2.4.2. Chemical Quality Analysis Methods
SiO2, dry residue, Ca2+, Mg2+, Fe (total), CO32-, HCO3-, SO42-, Cl-, NO2-, NO3-, total hardness, heavy metals: K, Na, Mn, Pb, Cu, Cr, Zn, Ni, As, Al and CO2, H2S, J, Br, B, organic matter content were determined using gravimetric, photometric, complexometric, argenometric, spectrophotometric, titrometric, and atomic adsorption methods.
3. Results and Discussion
The physical properties of water are determined by its state (solid, liquid, ice), molar mass, density, taste, odor, color, presence of turbidity or sediment, clarity, and water temperature [18-22]. For ease of analysis of 70 spring water sources, we conditionally named them from 1 to 70 (№1-№70). The physical properties of spring water were compared with the requirements of standards (UzDSt 950:2011 - “Drinking water” and UzO`U 0556:2012 - “Mineral drinking waters, medicinal natural waters”). According to them, the norm should be 0-2 points for taste, 0-2 points for smell, 0-2 mg/dm3 for turbidity, and 20 degrees for color. Since the sampling process from springs mainly coincided with the hot and warm season (May-September), their temperature was 16-18 oC. Of the spring water samples taken for the study, 69 were assessed as having an indicator of “0”, that is, odorless, and 69 as tasteless. Spring No. 16 received 2.1 points, which means it has an unpleasant smell, and a taste of 2.1 points, which means it has an unsatisfactory taste. The color level of the spring water samples was also calculated into points, according to which all the springs were transparent and clear. Spring waters can be consumed physically as drinking and medicinal mineral waters, with the exception of spring No. 16. The chemical indicators of water include pH, SiO2, dry residue, cations Ca2+, Mg2+, Fe (total), anions CO32-, HCO3-, SO42-, Cl-, NO2-, NO3-, total hardness, as well as the concentration of the most widespread pollutants from industrial and agricultural enterprises (heavy metals: K, Na, Mn, Pb, Cu, Cr, Zn, Ni, Co, As, Al) [18-21]. The healing properties of spring waters are determined by factors such as total mineralization, CO2, H2S, Fe (total), J, Br, B, SiO2, and the amount of organic matter [19].The pH indicators of the springs of the Gallaral district were analyzed during 2020-2023 and the following were revealed, namely No. 1, No. 4, No. 5, No. 6, No. 7, No. 8, No. 9, No. 10, No. 12, No. 13, No. 14, No. 16, No. 17, No. 18, No. 20, No. 21, No. 22, No. 23, No. 24, No. 25, No. 26, No. 27, No. 29, No. 30, No. 31, No. 32, No. 33, No. 34, No. 35, No. 36, No. 37, No. 38, No. 39, No. 41, No. 42, No. 44, No. 45, No. 46, No. 49, No. 50, No. 51, No. 52, No. 54, № 55, № 58, № 59, № 60, № 62, № 63, № 64, № 65, № 66, № 67, № 69, № 70 showed a weak alkaline environment, while spring waters № 2, № 3, № 11, № 15, № 19, № 28, № 40, № 43, № 47, № 48, № 53, № 56, № 57, № 61, № 68 showed a neutral environment. The dark red color in the graph represents the permissible norm.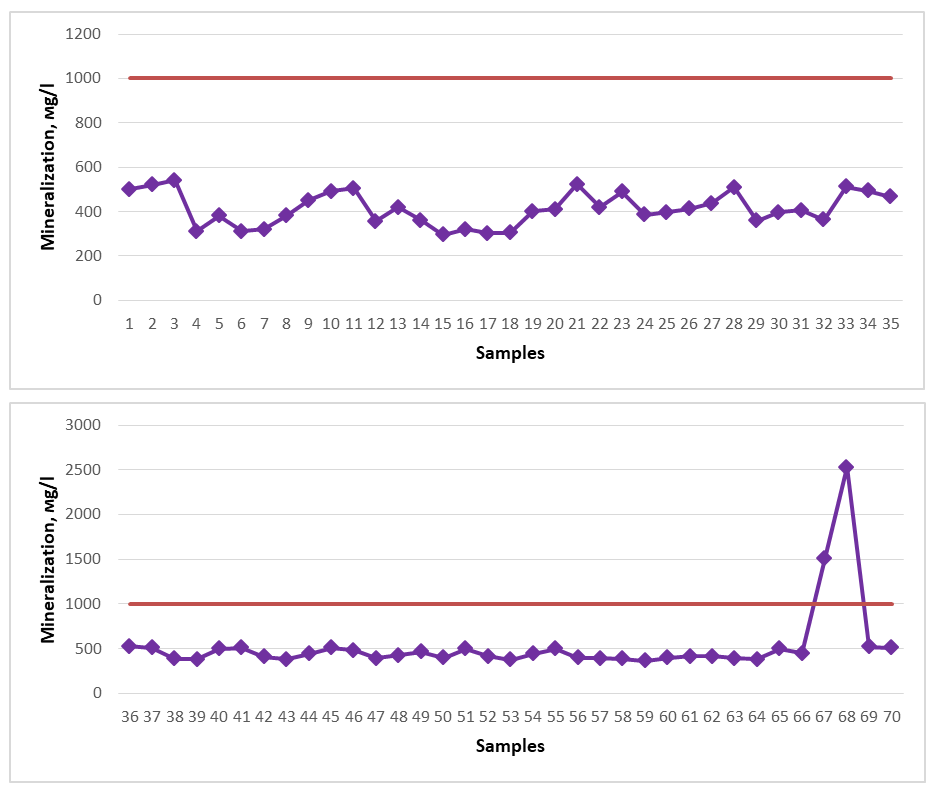 | Figure 1. Mineralization (2020-2023) |
The dry residue (total mineralization) of spring waters distributed in the territory of the Gallaral district should not exceed 1000.00-1500.00 mg/d3 for drinking water, and more than 1000.00 mg/d3 for medicinal mineral water. According to the requirements for medicinal mineral water, ˂1.0 g/l is considered weakly mineralized, 1.0-5.0 g/l is considered lowly mineralized, 5.0-10 g/l is considered normal mineralized, and 10-35 g/l is considered highly mineralized [19-21]. When testing spring waters taken as samples, all indicators of the results obtained are less than 1000.00 mg/d3. However, spring waters No. 67 and No. 68 do not meet the requirements for drinking water. In the graph, the dark red color indicates the permissible norm.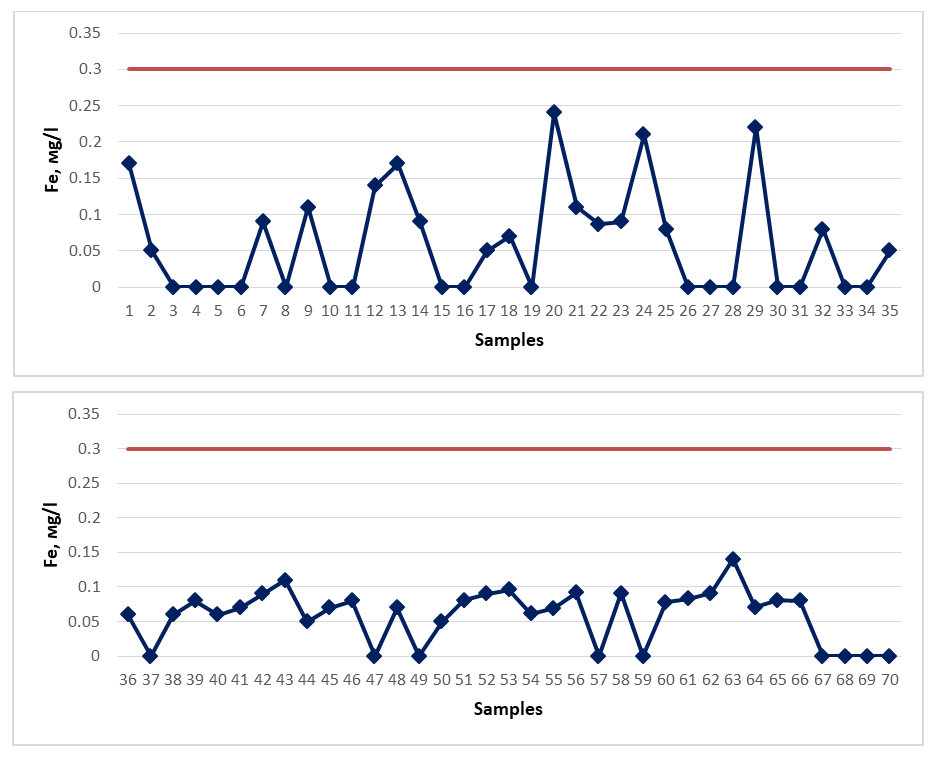 | Figure 2. Total ferrum (2020-2023) |
The total iron (Fe) content of spring waters distributed in the territory of the Gallaral district should not exceed 0.3 mg/d3 according to the requirement. When testing the spring waters taken as samples, all the results obtained meet the requirements for drinking water, that is, all indicators are less than 0.3 mg/d3. As for iron-rich healing mineral water, its content in the water should be at least 10 mg/d3. However, not all spring water samples tested for the study had a sufficient iron content and were not evaluated as healing mineral water. In the graph, the dark red color indicates the permissible norm.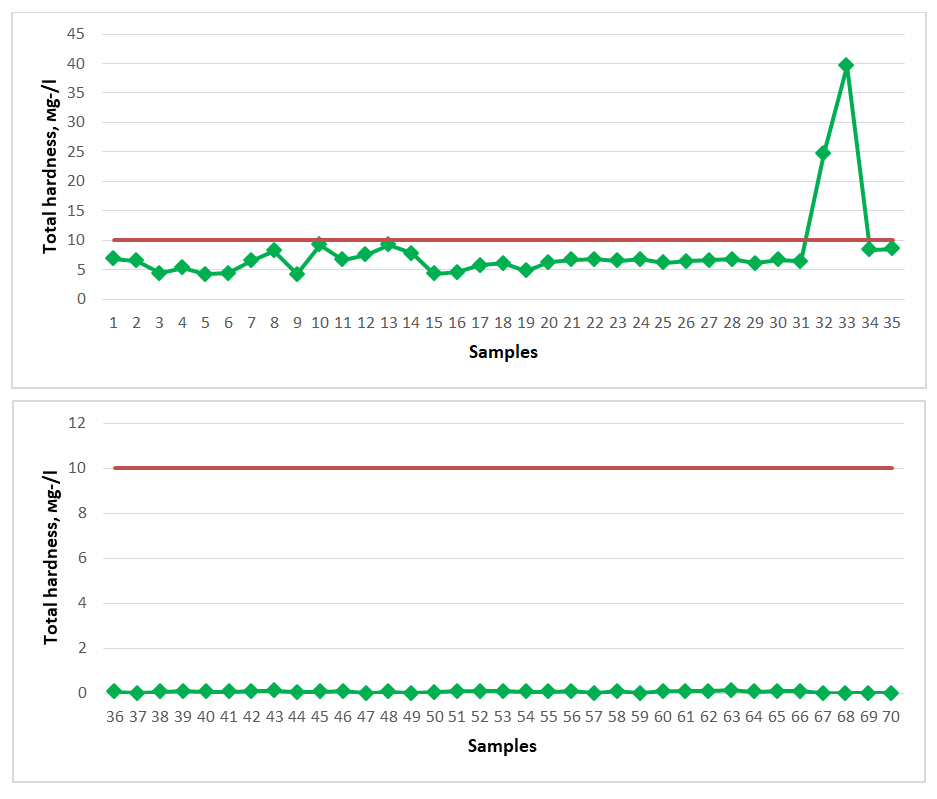 | Figure 3. Total hardness (2020-2023) |
The general hardness level of spring water resources distributed in the territory of Gallaorol district of Jizzakh region was checked and compared with the standard requirements. According to it, the general hardness in spring waters should not exceed 7.00-10.00 mg-eq/dm3. According to the results of the comparison, springs No. 32, No. 33 showed a general hardness of 24.8 mg-eq/dm3, 39.6 mg-eq/dm3, respectively. These springs are not recommended for drinking as drinking water, nor as healing mineral water; such waters are popularly called “hard waters”.The amount of silicon oxide (SiO2) in spring waters is not regulated by a specific indicator as drinking water, but as medicinal mineral water it should be 50.0 mg/dm3, and such waters are silicon waters and are used to treat diseases of the gastrointestinal tract, metabolism, musculoskeletal system, nervous system, and skin diseases. The amount of silicon oxide in spring waters distributed in the Gallaorol district of Jizzakh region is presented below (Figure 4). The dark red line in the graph represents the permissible norm.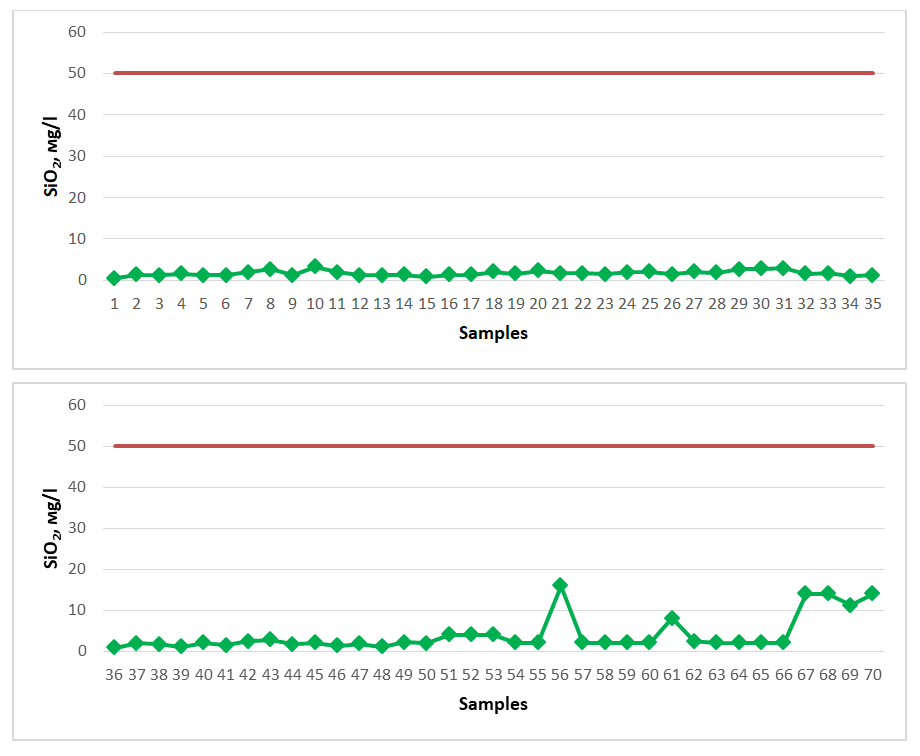 | Figure 4. SiO2 (2020-2023) |
Springs No. 1, No. 15, No. 34, No. 36 were found to have the lowest silicon oxide content, with amounts of 0.5 mg/dm3, 0.8 mg/dm3, 0.9 mg/dm3, and 0.8 mg/dm3, respectively. Springs No. 56, No. 67, No. 68, and No. 70 were found to have the highest silicon oxide content, with amounts of 16 mg/dm3, 14 mg/dm3, 14 mg/dm3, and 14 mg/dm3, respectively. Since the amount of this chemical compound in the spring waters is less than 50.0 mg/dm3, we cannot call them silicon-rich healing mineral waters, but they are recommended as sources of drinking water suitable for drinking.The high iodine content in spring water resources is used in medicine for the treatment of gastritis, intestinal diseases, liver, musculoskeletal diseases, skin, cardiovascular and peripheral nervous system, and gynecological diseases [17-20]. Its content in spring waters should be 5 mg/l. The iodine content in spring waters distributed in the Gallaorol district of Jizzakh region is shown below. All spring water samples studied for the study were found to have a low iodine content and did not meet the requirements for mineral medicinal water with respect to iodine. Among the springs, spring No. 55 showed the maximum indicator, 2.20 mg/l (Figure 5). The dark red line in the graph represents the permissible norm.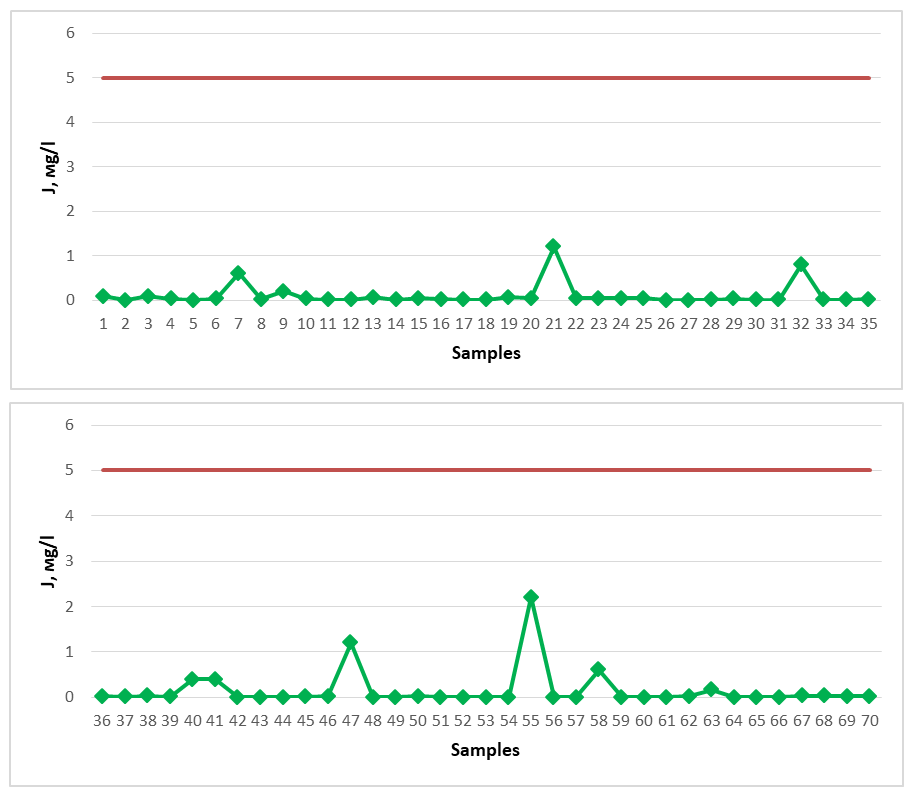 | Figure 5. Iodine (J) (2020-2023) |
The spring water resources of Gallaorol district of Jizzakh region do not contain a high amount of iodine, although they are not considered as medicinal mineral waters, since there is no specific standard for natural spring waters regarding iodine, these spring waters studied can be consumed as drinking water. If the chemical element bromine is present in large quantities in spring waters, such waters are considered brominated medicinal waters, and in medicine they are used to treat cardiovascular, nervous system, musculoskeletal system, and skin diseases [18-22]. As a medicinal mineral water, its content in water should be 25 mg/l. Water samples from springs of Gallaorol district were tested for bromine element (Figure 6). The dark red line in the graph represents the permissible norm.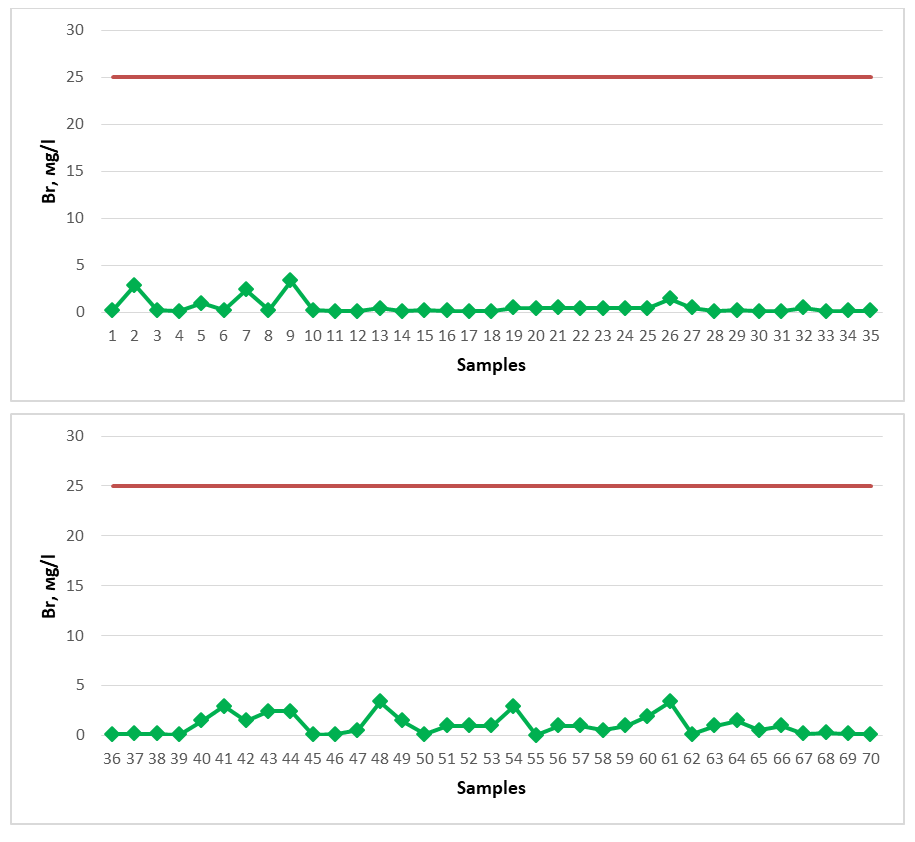 | Figure 6. Br (2020-2023) |
All spring water samples studied for the study were found to have a low bromine content and did not meet the requirements for mineral medicinal water with respect to bromine. Among the springs, springs No. 2 (2.88 mg/l), No. 7 (2.40 mg/l), No. 9 (3.36 mg/l), No. 41 (2.88 mg/l), No. 43 (2.40 mg/l), No. 44 (2.40 mg/l), No. 48 (3.36 mg/l), No. 54 (2.88 mg/l), No. 61 (3.36 mg/l) showed the maximum indicator, 2.20 mg/l. The spring water resources of Gallarol district of Jizzakh region do not contain a high amount of bromine, although they are not considered as medicinal mineral waters, since there is no specific norm for natural spring waters regarding bromine, these spring waters studied can be consumed as drinking water.If the chemical element boron is present in a large amount in spring waters, such waters are considered medicinal waters with boron, and in medicine they are used to treat diseases of the digestive system [19-22]. As a medicinal mineral water, its content in water should be 35 mg/l. Water samples from springs of Gallarol district were tested for boron (Figure 7). The dark red line in the graph represents the permissible norm.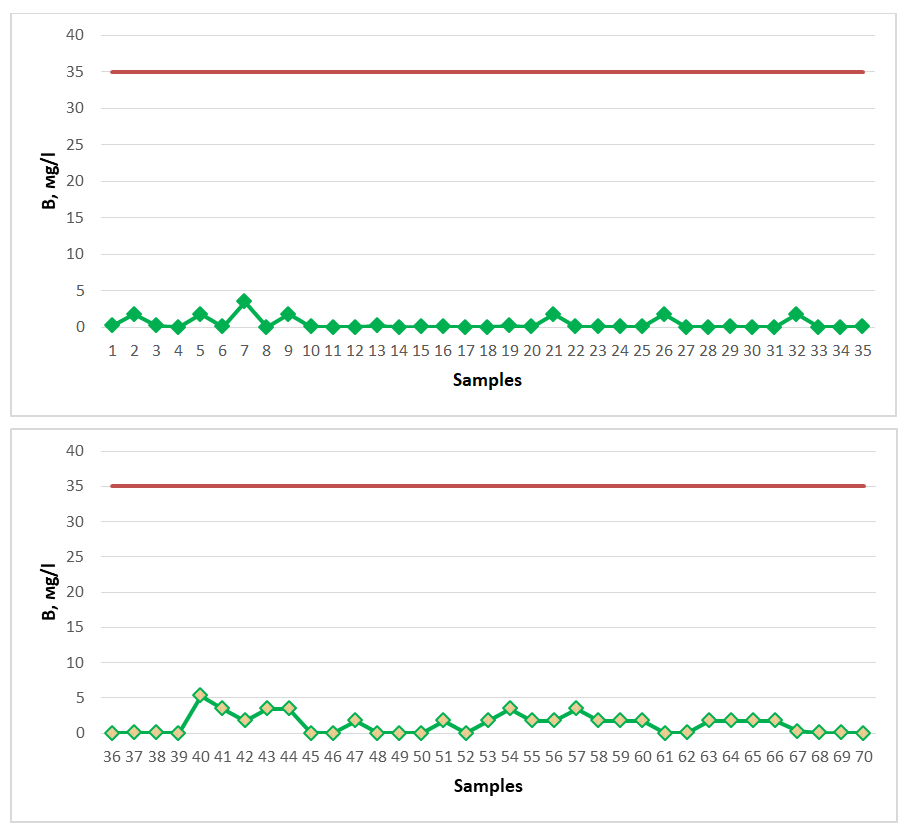 | Figure 7. B (2020-2023) |
All of the spring water samples studied for the study were found to have a low boron content and were proven not to meet the requirements for mineral medicinal water with respect to boron. Among the springs, springs No. 7 (3.47 mg/l), No. 40 (5.30 mg/l), No. 41 (3.47 mg/l), No. 43 (3.47 mg/l), No. 44 (3.47 mg/l), No. 54 (3.47 mg/l), No. 57 (3.47 mg/l) showed the maximum indicator (Figure 7). The content of boron in the spring water resources of the Gallaorol district of the Jizzakh region is not high, although they are not considered medicinal mineral waters, since there is no specific norm for natural spring waters with respect to boron, these spring waters studied can be consumed as drinking water.If the CO2 content of spring waters is 0.5-1.4 g/l, such water is considered weakly carbonated healing water. If it is 1.4-2.5 g/l, it is considered to be normal-concentrated carbonated healing water. If it is >2.5 g/l, it is considered to be strongly carbonated (carbonated) healing water, which is used in medicine for digestion and the treatment of cardiovascular diseases [19-21]. Therefore, for carbonic anhydride to be considered medicinal spring water, its content must be at least 0.5 g/l (500 mg/l). Water samples from springs in the Gallaral region were tested for CO2 (Figure 8). The dark red line in the graph represents the permissible norm.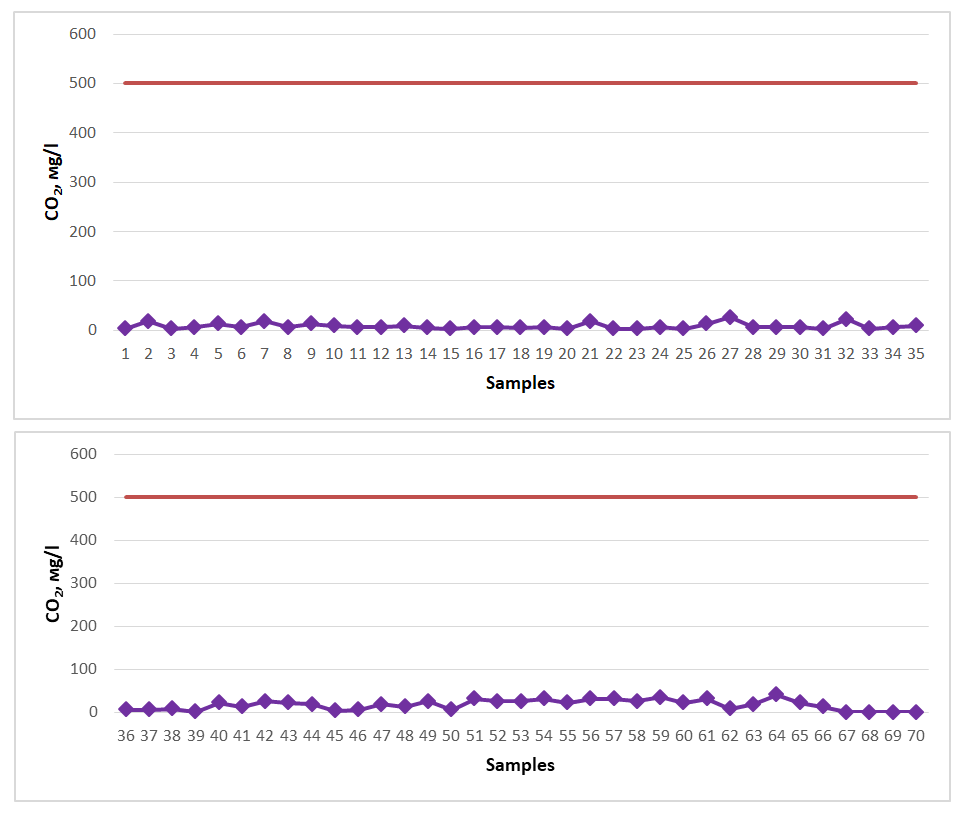 | Figure 8. CO2 (2020-2023) |
All of the spring water samples examined for the study were found to have low levels of CO2 and were proven not to meet the requirements for mineral healing water with respect to CO2. Among the springs, No. 27 (26.0 mg/l), No. 32 (22.0 mg/l), No. 40 (22.0 mg/l), No. 42 (26.0 mg/l), No. 43 (22.0 mg/l), No. 49 (26.0 mg/l), No. 51 (31.0 mg/l), No. 52 (26.0 mg/l), No. 53 (26.0 mg/l), No. 54 (31.0 mg/l), No. 55 (22.0 mg/l), No. 56 (31.0 mg/l), No. 57 (31.0 mg/l), No. 58 (26.0 mg/l), No. 59 (35.0 mg/l), No. 60 (22.0 mg/l), No. 61 (31.0 mg/l), No. 64 (40.0 mg/l), No. 65 (22.0 mg/l) springs showed the maximum indicator. The content of CO2 in the spring water resources of Gallaorol district of Jizzakh region is not high, although they are not considered as medicinal mineral waters, since there is no specific norm for natural spring waters in terms of CO2, these studied spring waters can be consumed as drinking water.If the content of H2S (total H2S + HS-) in spring waters is 10.0-50 mg/l, such water is considered to be weakly hydrogen sulfide medicinal water. If it is 50.0-100.0 mg/l, it is considered to be medium-concentration hydrogen sulfide medicinal water. If the concentration of hydrogen sulfide is 100.0-250.0 mg/l, it is considered a strong hydrogen sulfide healing water, and if it is > 250 mg/l, it is considered a very strong hydrogen sulfide healing water, and in medicine they are used to treat diseases such as cardiovascular, nervous, gynecological, skin, and radiculitis [16-18]. Therefore, for hydrogen sulfide to be considered a healing spring water, its concentration must be at least 10 mg/l. Water samples from springs in the Gallaral region were tested for H2S (total H2S+HS-) (Figure 9). The dark red line in the graph represents the permissible norm.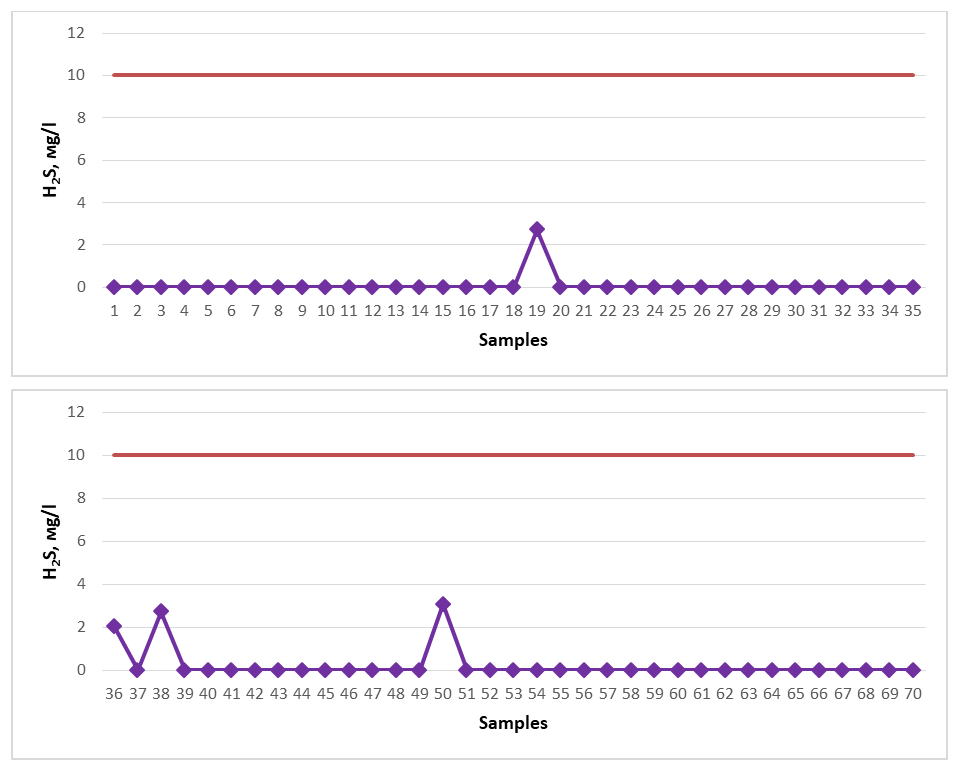 | Figure 9. H2S (2020-2023) |
All spring water samples studied for the study were found to contain a low amount of H2S (total H2S+HS-) and were proven not to meet the requirements for mineral medicinal water with respect to H2S (total H2S+HS-). Among the springs, springs No. 19 (2.72 mg/l), No. 36 (2.04 mg/l), No. 38 (2.72 mg/l), No. 50 (3.06 mg/l) showed the maximum indicator. The content of H2S (total H2S+HS-) in the spring water resources of Gallaorol district of Jizzakh region is not high, although they are not considered medicinal mineral waters, since there is no specific standard for natural spring waters with respect to H2S (total H2S+HS-), these spring waters studied can be consumed as drinking water.If the content of organic substances in spring water is 5.0 mg/l, then such water is considered to be healing water rich in organic substances, and in medicine it is used to treat digestive, liver, metabolic, and urogenital diseases [16-21]. Therefore, for it to be healing spring water with organic composition, its content should be at least 5.0 mg/l. Water samples from springs in the Gallaral district were tested for the content of organic substances (Figure 10). The dark red line in the graph represents the permissible norm.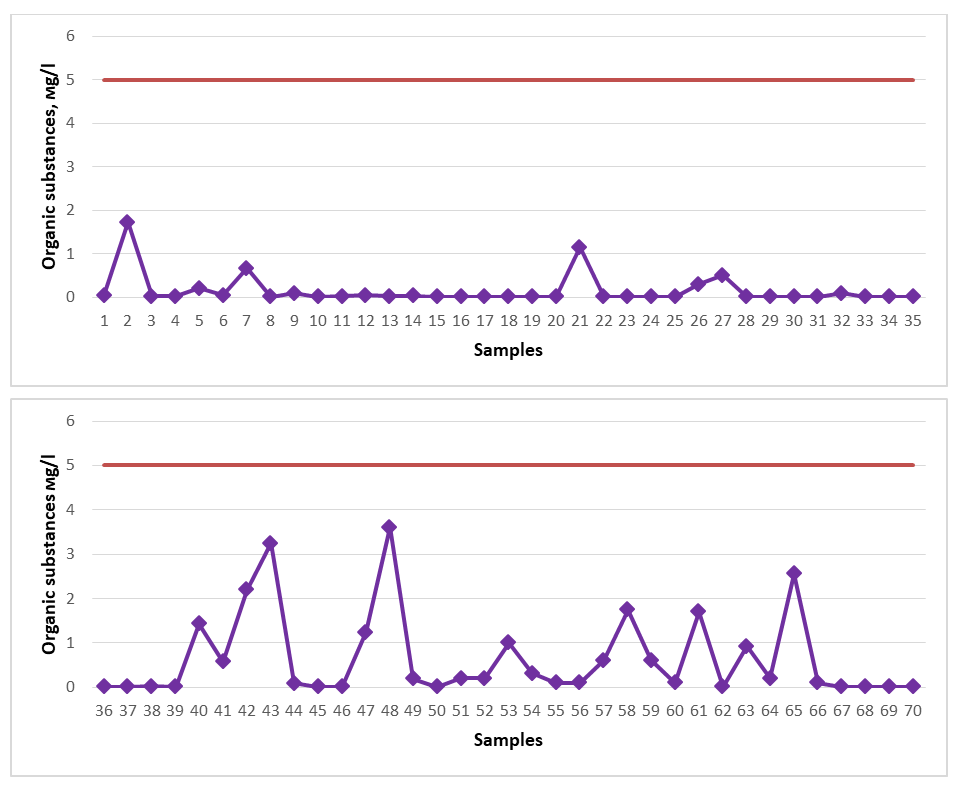 | Figure 10. Organic matter (2020-2023) |
The content of organic substances in the water of the springs did not meet the quality requirements for healing mineral water rich in organic substances, that is, the highest indicators were shown by No. 2 (1.72 mg/l), No. 21 (1.15 mg/l), No. 40 (1.43 mg/l), No. 42 (2.20 mg/l), No. 43 (3.25 mg/l), No. 47 (1.24 mg/l), No. 48 (3.60 mg/l), No. 58 (1.75 mg/l), No. 61 (1.70 mg/l), No. 65 (2.56 mg/l). From the results of this analysis, it is clear that although the spring waters do not meet the quality requirements for healing mineral water in terms of organic composition, they nevertheless meet the requirements for drinking water quality.
4. Conclusions
The physical and chemical parameters of the spring waters (pH, SiO2, dry residue, Ca2+, Mg2+, Fe (total), CO32-, HCO3, SO42-, Cl-, NO2-, NO3-) were analyzed. According to them, out of the 70 spring water resources studied, 9 (13%) (Kichik Bulok (No. 16), Auliya Bulok (No. 21), Koktol (No. 24), Tuvadok (No. 32), Aktayak (No. 33), Buloknur (No. 67), Sunnatbulok (No. 68), Umr Baba (No. 69), Eski Dokan-Aldi Bulok (No. 70)) were found to be unsuitable for drinking. However, all the remaining 61 springs (87%) were found to be organoleptically high-quality, potable waters. In addition, it was found that not all spring waters distributed in the region correspond to the healing properties of mineral water.
References
| [1] | Belval, L.N.; Hosokawa, Y.; Casa, D.J.; Adams, W.M.; Armstrong, L.E.; Baker, L.B.; Burke, L.M.; Cheuvront, S.N.; Chiampas, G.; González-Alonso, J.; et al. Practical Hydration Solutions for Sports. Nutrients 2019, 11, 1550. [Google Scholar] [CrossRef] [Green Version]. |
| [2] | Alberts et al. Total luminescence spectra of IHSS standards and reference fulvic acids, humic acids and natural organic matter: comparison of aquatic and terrestrial source terms. Organic Geochemistry. (2004). |
| [3] | A. Baker et al. Fluorescence wavelength and intensity variations of cave waters Journal of Hydrology. (1999). P. 132. |
| [4] | D.J. Burdige et al. Fluorescent dissolved organic matter in marine sediment pore waters Marine Chemistry. (2004). P. 145. |
| [5] | Abbott G.D., Fletcher I.W., Tardio S., and Hack E. (2017) Exploring the geochemical distribution of organic carbon in early land plants: a novel approach. Philos Trans R Soc Lond B Biol Sci 373. |
| [6] | Braunstein D. and Lowe D.R. (2001) Relationship between spring and geyser activity and the deposition and morphology of high temperature (>73°C) siliceous sinter, Yellowstone National Park, Wyoming, U.S.A. Journal of Sedimentary Research 71: 747–763. |
| [7] | Campbell K.A., Lynne B.Y., Handley K.M., Jordan S., Farmer J.D., Guido D.M., Foucher F., Turner S., and Perry R.S. (2015) Tracing biosignature preservation of geothermally silicified microbial textures into the geological record. Astrobiology 15: 859–882. |
| [8] | A.I.Abd El-Mageed, A.H .El-Kamel, A.B.Abbady, Sh.Harb, I. I.Saleh. Natural radioactivity of ground and hot spring water in some areas in Yemen. Desalination. Volume 321, 15 July 2013, Pages 28-31. |
| [9] | E.O. Agbalagba et al. Evaluation of natural radioactivity in soil, sediment and water samples of Niger Delta (Biseni) Flood Plain Lakes, Nigeria. J. Environ. Radioact. (2011). P. 72. |
| [10] | J.M. Godoy et al. Natural radioactivity in Brazilian groundwater. J. Environ. Radioact. (2006). P. 87. |
| [11] | S. Labidi et al. Natural radioactive nuclides in some Tunisian thermo-mineral springs. J. Environ. Radioact. (2002). P. 123. |
| [12] | S.A. Saqan et al. Radionuclides in hot mineral spring waters in Jordan. J. Environ. Radioact.(2001). P. 188. |
| [13] | N.K. Ahmed. Natural radioactivity of ground and drinking water in some areas of upper Egypt. Turk. J. Eng. Environ. Sci. (2004). P. 74. |
| [14] | Seung-Gu Lee et. al. Strontium isotope geochemistry and its geochemical implication from hot spring waters in South Korea. Journal of Volcanology and Geothermal Research. Volume 208, Issues 1–2, 1 November 2011, Pages 12-22. |
| [15] | J. Du et al. Variations of geothermometry and chemical fluids in the Rehai geothermal field, southwestern China. Journal of Volcanology and Geothermal Research. (2005). Pages 43-52. |
| [16] | D.M. Han et al. Evaluation of groundwater hydrochemical characteristics and mixing behavior in the Daying and Qicun geothermal systems, Xinzhou Basin. Journal of Volcanology and Geothermal Research. (2010). Pages 12-24. |
| [17] | Y. Lee et al. Geothermal resource assessment in Korea. Renewable & Sustainable Energy Reviews. (2010). Pages 22-42. |
| [18] | P. Möller et al. Rare earth elements, yttrium and Pb isotope ratios in thermal spring and well waters of West Anatolia, Turkey: a hydrochemical study of their origin. Chemical Geology. (2004). P. 102. |
| [19] | P. Négrel et al. Storntium isotope systematic used to decipher the origin of groundwaters sampled from granitoids: the Vienne Case (France). Chemical Geology. (2001). P. 26-42. |
| [20] | T. Nakano et al. Determination of seasonal and regional variation in the provenance of dissolved cations in Japan based on Sr and Pb isotopes. Atmospheric Environment. (2006). P. 201. |
| [21] | D.C. Papp et al. Isotopic composition and origin of mineral and geothermal waters from Tuşnad Băi Spa, Harghita Mountains, Romania. Journal of Geochemical Exploration. (2006). Pages 102-127. |
| [22] | D. Qin et al. Hydrogeochemistry and groundwater circulation in the Xi'an geothermal field, China Geothermics (2005). P. 43-56. |
| [23] | S.-S. Park et al.Temperature evaluation of the Bugok geothermal system, South Korea Geothermics (2006). P. 44-67. |












 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML