-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(9): 190-192
doi:10.5923/j.ijge.20251309.05
Received: Aug. 28, 2025; Accepted: Sep. 19, 2025; Published: Sep. 29, 2025

Study of General for Clinical Toxicology Strains Bacteria Bacillus Amyloliquefaciens-UzMU 22
Luiza Tagayeva1, Shermat Jabborov2, Kunduz Normurodova1
1Department of Biotechnology and Microbiology, Faculty of Biology and Ekology, National University of Uzbekistan, Tashkent, Uzbekistan
2Scientific Research Institute of Livestock and Poultry, Tashkent, Uzbekistan
Correspondence to: Luiza Tagayeva, Department of Biotechnology and Microbiology, Faculty of Biology and Ekology, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The cultural-morphological properties and enzyme-forming ability of endophytic bacterial isolate isolated from the medicinal plant Kalanchoe degremania were studied. In this case, glucoamylase activity was 6.2 units/ml and protein activity 1.2 units/mg, cellulose activity 1.5 units/ml and protein activity 0.4 units/mg, xylanase activity 0.6 units/ml and protein activity 0.32 units/mg. It has shown high antagonistic properties to the pathogenic fungus Candida albicans, pathogenic bacteria such as Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa. Sensitivity to various antibiotics was also tested. The selected active isolate was identified by 16C RNA and registered in GenBank under the number OQ349559 and named Bacillus amyloliquefaciens-UzMU 22. The culture fluid of the strain Bacillus amyloliquefaciens-UzMU 22 was tested orally and on the skin of rats, the cumulative effect and acute toxicological properties of the local pathogen in mice were studied. It was established that this bacterial strain is non-toxic.
Keywords: Microorganism, Bacillus subtilis, Strain, Bacteria, Cumulative, Toxicology, Culture liquid, Enzyme, Probiotic, Protein
Cite this paper: Luiza Tagayeva, Shermat Jabborov, Kunduz Normurodova, Study of General for Clinical Toxicology Strains Bacteria Bacillus Amyloliquefaciens-UzMU 22, International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 190-192. doi: 10.5923/j.ijge.20251309.05.
1. Introduction
- In recent years, large-scale studies have been actively conducted aimed at isolating endophytic bacteria from medicinal plants and studying their probiotic properties. These bacteria are considered as a promising direction for use as additional components in the production of feed enriched with probiotics. Their use helps maintain normal microflora in the digestive system of farm animals, which, in turn, can improve digestion, increase resistance to diseases and contribute to the overall health of animals [1-6].Probiotics are preparations made from live microorganisms that, when introduced into the human or animal body, have a positive effect on the physiological, biochemical and immune responses of the host organism by normalizing the composition of the intestinal microflora [2,5-6]. Due to these properties, probiotics are widely used in medicine and veterinary science as an alternative or supplement to antibiotic therapy, as well as for the prevention and treatment of gastrointestinal diseases. The growing need for more effective, stable and broad-spectrum antagonist biopreparations has led to the development of second-generation probiotics [9-11]. Second generation probiotics are produced primarily on the basis of spore-forming bacteria of the genera Bacillus, Clostridium and Brevibacillus, which are highly resistant to adverse environmental conditions and remain viable during storage and transportation [5,9]. These probiotics demonstrate high antagonistic activity against pathogenic and opportunistic microorganisms, stimulate the growth of beneficial microflora and improve the overall health of the body. In this regard, active research is being conducted on their use not only for therapeutic purposes, but also as part of enriched feed for farm animals. Previously, we studied the probiotic properties of the bacterium strain Bacillus amyloliquefaciens-UzMU 22 [6].The aim of this work is to study the general toxicology of the bacterial strain Bacillus amyloliquefaciens-UzMU 22.The objectives of the study included: 1. Determination of acute toxicity upon oral and cutaneous administration. 2. Study of cumulative action (subacute toxicity) on mice. 3. Determination of local irritant action.
2. Materials and Methods
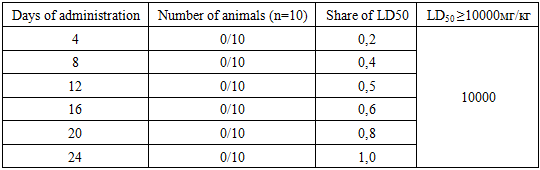
- Bacillus amyloliquefaciens-UzMU 22 bacteria was transferred from the National University of Uzbekistan to the testing laboratory for quality control and circulation of veterinary drugs and feed additives at the “State Scientific Center for Quality Control and Circulation of Veterinary Drugs and Feed Additives” for preclinical studies of general toxicology.The general effect and “acute” toxicity of Bacillus amyloliquefaciens-UzMU 22 were determined on 10 white outbred mice and rats of both sexes weighing 19-21±2 and 200-280±10 g after a single oral administration. The Litchfield and Wilcoxon method [7-8] was used to determine the parameters of “acute” toxicity, and the Noakes and Sanderson method for cutaneous application was used with 10 animals per group [8]. The study of the accumulation of Bacillus amyloliquefaciens-UzMU 22 was carried out using the Lim,a et al. method on 10 mice [8]. The local irritant effect of Bacillus amyloliquefaciens-UzMU 22 was assessed by the effect of the bacteria on the skin of 10 rats and the mucous membrane of the eye of 6 rabbits [8].The Litchfield and Wilcoxon method was used to determine the parameters of "acute" toxicity upon oral administration. Each dose of the substance was tested on 6 animals. Observation was carried out for 14 days. Bacillus amyloliquefaciens-UzMU 22 was administered orally at doses of 2000, 4000, 5000, 6000, 8000 and 10000 mg/kg.A study of the general action of Bacillus amyloliquefaciens-UzMU 22 showed that at doses of 4000-6000 mg/kg, the behavior of mice and rats did not differ from intact animals. At doses of 5000 and 10000 mg/kg, animal inhibition was noted for 120-180 minutes. No animal deaths were observed for 14 days. At single, skin application cultural liquid (CL) of bacteria treated on the cut-off site of skin of rats’ back and sides of 35 cm2, to mice of 3,5 cm2 after single putting solutions in a dose of 10 ml/kg of animals placed in separate cages, a skin site with the studied substance left open, supervision was conducted hourly in day of introduction, by 3 times a day for 2-3 days and once a day in the next 7-10 days of experience.General behavior, skin condition at the site of application of the liquid, presence of redness, fur condition, motor activity and death of rats were taken into account. Observation was carried out for 14 days. If substances for external use do not exhibit toxic effects when studying acute toxicity at a dose of 10 ml/kg, then such agents are considered non-toxic.Thus, the study of the general action and “acute” toxicity of Bacillus amyloliquefaciens-UzMU 22 showed that this bacterial strain belongs to class V of practically non-toxic compounds. LD50 - more than 10,000 mg/kg oral administration in mice and rats does not cause death of animals at a dose of 10 ml/kg when applied topically.The study of the accumulation of Bacillus amyloliquefaciens-UzMU 22 was conducted using the method of Lim, a and others, which allows us to evaluate not only accumulation, but also habituation. The objective of the study was to identify the possible cumulative property of Bacillus amyloliquefaciens-UzMU 22. The experiments were conducted on 10 mice of both sexes weighing 19-21±2 g. The CL was administered orally according to the following scheme (Table 1).
|
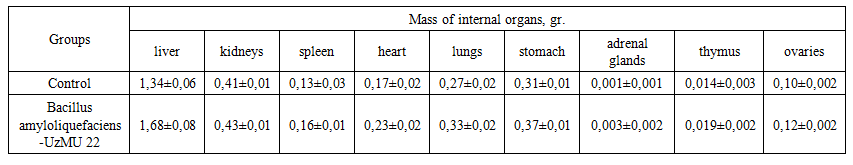 | Table 2. Average values of internal organ weights in mice after oral administration of Bacillus amyloliquefaciens-UzMU 22 (M±m; n=10; P> 0.05) |
3. Conclusions
- Thus, the studied strain Bacillus amyloliquefaciens-UzMU 22 is a non-toxic strain that can be recommended for feed additives in livestock farming.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML