-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(9): 169-172
doi:10.5923/j.ijge.20251309.01
Received: Aug. 20, 2025; Accepted: Sep. 12, 2025; Published: Sep. 16, 2025

Effect of Experimental Diabetes on the Carbohydradases and Oligopeptide Hydrolases Activity in the Small Intestine in Rats
Kuchkarova Lubov Salijanovna1, Qiyomova Nozigul Farxod qizi2, Kayumov Khasan Yusuf ogli1, Achilov Rashidbek Khudayberganovich1
1National University of Uzbekistan, Tashkent, Uzbekistan
2Karshi State University, Karshi, Uzbekistan
Correspondence to: Kayumov Khasan Yusuf ogli, National University of Uzbekistan, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

It is known that the small intestine is a part of the digestive tract where hydrolysis and absorption of most nutrients, including carbohydrates and proteins, occurs. In this work, it is shown that diabetes mellitus can significantly increase the hydrolysis of oligosaccharides, especially in the proximal and medial parts of the small intestine, against the background of unchanged expression of oligopeptide hydrolysis. This was manifested in the fact that in diabetes mellitus, the activity of intestinal carbohydrases (glucoamylase, maltase, sucrase and lactase) increased, and the activity of oligopeptide hydrolase (glycyl-L-leucine dipeptide hydrolase and diglycyl-L-leucine tripeptide hydrolase) remains at the same level as in healthy animals. Therefore, diabetes has a specific effect on the hydrolysis of proteins and carbohydrates in the small intestine. These data suggest that inhibitors of small intestinal carbohydrase activity may be potential antidiabetic agents.
Keywords: Rats, Alloxan diabetes, Small intestine, Carbohydrases, Oligopeptide hydrolases
Cite this paper: Kuchkarova Lubov Salijanovna, Qiyomova Nozigul Farxod qizi, Kayumov Khasan Yusuf ogli, Achilov Rashidbek Khudayberganovich, Effect of Experimental Diabetes on the Carbohydradases and Oligopeptide Hydrolases Activity in the Small Intestine in Rats, International Journal of Genetic Engineering, Vol. 13 No. 9, 2025, pp. 169-172. doi: 10.5923/j.ijge.20251309.01.
Article Outline
1. Introduction
- Diabetes mellitus (DM) is a progressive chronic endocrine disease characterized by increased blood glucose levels due to insulin deficiency or decreased insulin response. It is a severe metabolic disease characterized by impaired carbohydrate, fat, protein and other types of metabolism. Impaired carbohydrate and protein metabolism are primarily associated with the inability of tissues and cells to absorb glucose in diabetes. This leads to a decrease in the rate of protein synthesis and increased breakdown, since the body uses proteins for gluconeogenesis [1,2]. The initial stage of carbohydrate and protein metabolism occurs mainly in the small intestine, the most important part of the digestive tract, where hydrolysis and absorption of about 90% of carbohydrates and proteins occurs [3-6]. Mechanically and partially chemically processed food polymers entering the small intestine under the action of enzymes of pancreatic, hepatic and intestinal juices begin to split into oligomers. Then the oligomers are split by membrane-bound enzymes of the brush border into monomers, which can enter the bloodstream and satisfy the needs of cells for plastic and energy material [4-7]. Although food hydrolysis in the small intestine is the initial stage of digestion of food polymers and plays a key role in providing the body with nutrients for plastic and energy metabolism, the assimilation of carbohydrates and proteins in the small intestine in diabetes mellitus has been almost unstudied.In this regard, the purpose of the work is to study the effect of experimental diabetes on the activity of small intestine сarbohydrases and oligopeptide hydrolases.
2. Materials and Methods
- For this experiment outbred white male rats weighing 150–180 g was kept in standard vivarium condition with unlimited access to water and food. The experiments were conducted according to the Helsinki Declaration of the World Medical Association 2010. Rats were divided into control and experimental diabetic groups. Diabetes in rats was induced by intraperitoneal injection of alloxan monohydrate (Сhеmlaborreaktiv LLC, Ukraine) solution in saline at a dose of 50 mg/kg/24 h for 3 days. Control group of rats was treated with saline by the same way.The glucose level was determined one week after the last injection of alloxan. For this, the tip of the rat's tail was cut off with sharp surgical scissors, and a special strip with filter paper was applied to the released drop of blood. The strip was transferred to a special cell of the glucometer (Accu-Chek Active, Germany). The display immediately showed the amount of glucose in mmol/l. Rats with a blood glucose level of more than 15 mmol/l were considered diabetic and were used for further observation. Experimental tests began 10 days after the last injection of alloxan.After rat decapitation, the small intestine was removed from the abdominal cavity, cleared of fatty tissue, divided into 3 sections (proximal, medial and distal) and each section was washed with 5 ml of Ringer's solution (pH 7.2). The mucosa of each small intestine section was separated with a plastic spatula, weighed and diluted with Ringer's solution in a ratio of 1:9. The separated mucosa was homogenized, centrifuged, and the supernatant was analysed for disaccharides and oligopeptide hydrolases activity. All operations were carried out in cold conditions. The activity of glucoamylase, maltase, sucrase, and lactase were determined by the glucose oxidase method Dahlquist method [8]. The glycyl-L-leucine dipeptide hydrolase and diglycyl-L-leucine tripeptide hydrolase activity was determined by Tarvid and Kushak method [9]. Enzyme activity was expressed in μmol of glucose (for disaccharidases) or glycine (for oligopeptide hydrolases) per minute per gram of wet weight of tissue. The results were processed using the Origin 6.1 program. The arithmetic mean (M), standard error (±m), and statistical significance (P) were studied. Results were considered statistically significant if P was <0.05.
3. Results
- The ability of the small intestine to digest carbohydrates was studied on the basic of changes in the activity of glucoamylase, maltase, sucrase and lactase and the ability of the intestine to hydrolyse oligopeptides was analysed on the basic of the changes in the activity of glycyl-L-peptide hydrolase and diglycyl-L-leucine tripeptide hydrolase in control and diabetic rats.
3.1. Activity of Carbohydrases
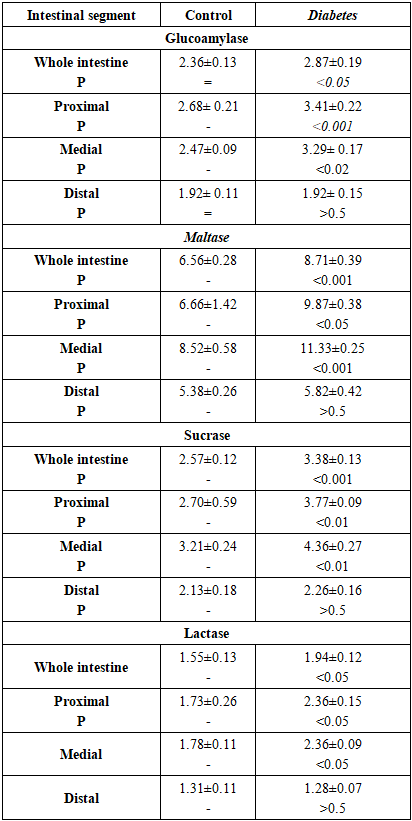
- Data on the effect of diabetes mellitus on the activity of intestinal carbohydrases are presented in Table 1.
|
3.2. Activity of Oligopeptide Hydrolases
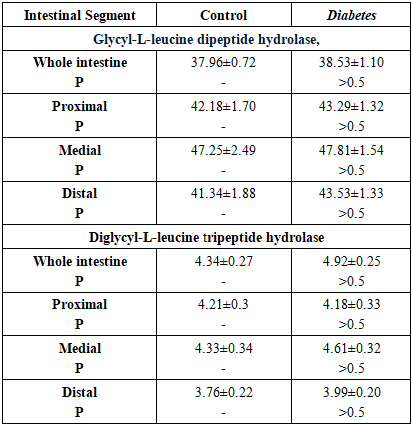
- The results of the manifestation of oligopeptide hydrolases activity in healthy rats and in rats with diabetes mellitus are presented in Table 2.
|
4. Discussion
- It is known that diabetes can be caused by insufficient insulin production by the pancreas β-cells (type 1 diabetes) or decreased sensitivity of cells to insulin (type 2 diabetes). The body cells are unable to assimilate glucose effectively, which leads to an excess of glucose in the blood against the background of an acute deficiency of the monomer in other tissues [6,10]. The source of exogenous glucose is mainly poly- and oligosaccharides, which are broken down in the small intestine by disaccharidases to monosaccharides, the main one of which is glucose [6,7]. The data showed that rats with diabetes mellitus had a greater ability to transport glucose from the small intestine into the blood than healthy rats. This is evidenced by the fact that in diabetic rats, the activity of glucoamylase, maltase, sucrase and lactase is increased in the intestinal mucosa compared to healthy animals, especially in the proximal and distal parts of the intestine. Diabetes-dependent structural-biomechanical remodeling of microflora, topography of autonomic and enteric neuropathy, smooth muscle dysfunction in the intestines of animals and humans have also been noted [11,12,13]. Based on such disturbances in the structural and functional topography of the intestine, it can be assumed that they are interconnected and lead to impaired motility, absorption and secretion and other gastrointestinal complications.Significant increase in α-glucosidase activities was also observed in diabetic patients as well as in diabetic rats [14,17,18]. Liu et al. showed that induction of sucrase-isomaltase complex activity is accompanied by high mRNA expression and increased protein content of this complex in the intestinal epithelium of rats with streptozotocin diabetitis. Insulin administration resulted in smoothing of diabetes-associated increase in the activity of these α-glucosidases. Furthermore, in a culture of Caco-2 cells, which imitate epithelial cells of the small intestine, insulin suppressed disaccharidase activity and expression of the sucrase-isomaltase mRNA complex, as well as the level of Cdx2 protein, which plays a key role in the development of intestinal epithelium. Based on these data, the authors suggest that diabetes-dependent increased sucrase and maltase activity is most likely due to insulin deficiency [18].We hypothesize that the observed increase in small intestinal carbohydrase activity is one of the significant causes of postprandial hyperglycemia, common in diabetes mellitus [2]. In this work, unlike others, it was found that diabetes mellitus, in addition to the activity of α-glucosidases (glucoamylase, maltase, sucrase) causes an increase in the activity of β-galactosidases (lactase) of the small intestine, which is also involved in the delivery of glucose from the small intestine into the blood. So, it is reasonable to consider intestinal carbohydrases as targets for the treatment of diabetes. The obtained data show that diabetes mellitus has a specific effect on the hydrolysis of carbohydrates and proteins in the small intestine. Despite the expression of intestinal disaccharidases in diabetic rats, the activity of both oligopeptidases remained at the level of healthy animals, i.e. the ability to digest oligopeptides in animals with diabetes mellitus did not differ from that in healthy animals. This was manifested in the fact that the activity of glycyl-L-leucine dipeptide hydrolase and diglycyl-L-leucine tripeptide hydrolase were expressed at a similar level with the control animals in the mucosa of the small intestine. Consequently, although the effect of diabetes on the intermediate metabolism of proteins, which is manifested in accelerated protein catabolism and is aimed primarily at replenishing the glucose deficiency in tissues, does occur [19], the assimilation of proteins in the small intestine is not impaired. Preservation of the intestine's ability to digest proteins at the level of healthy animals once again confirms the advisability of using inhibitors of intestinal carbohydrase activity for the prevention and treatment of diabetes mellitus.Thus, the ability of the small intestine to digest carbohydrates, but not proteins, increases in diabetes. Therefore, the activity of small intestinal disaccharidases may be a potential target for the correction of diabetes mellitus and other forms of hyperglycemia using inhibitors of intestinal carbohydrases.
5. Conclusions
- Thus, diabetes mellitus has an ambiguous effect on the activity of disaccharidases and oligopeptidases of the small intestinal mucosa in rats. The activity of disaccharidases increases, while the activity of oligopeptidases does not change. Diabetes-dependent increasing of activity is noted. as for α-glucosidase (glucoamylase, maltase, sucrase) and β-galactosidases (lactase) This increase in disaridase activity in diabetes is especially noticeable in the proximo-medial parts of the small intestine. Therefore, diabetes-dependent expression of intestinal carbohydrases activity in the intestinal tube must be taken into account in the treatment of diabetes and, possibly, in other forms of hyperglycemia.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML