-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2025; 13(8): 152-158
doi:10.5923/j.ijge.20251308.02
Received: Jul. 28, 2025; Accepted: Aug. 19, 2025; Published: Aug. 30, 2025

Ecological Characteristics of Phytophagous Plants of the Onion Agrocenosis of Uzbekistan
Abdullaev Ikram1, 2, Jumanazarov Hasanboy1, Abduraxmanov Dilmurod1, Iskandarov Abdulla1, 2, Aminov Muxammad1, Sultanbaeva Jamila1
1Khorezm Academy of Mamun, Khiva, Markaz str.1, Uzbekistan
2Urgench State University, Urgench, Khamid Alimjan str.14, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

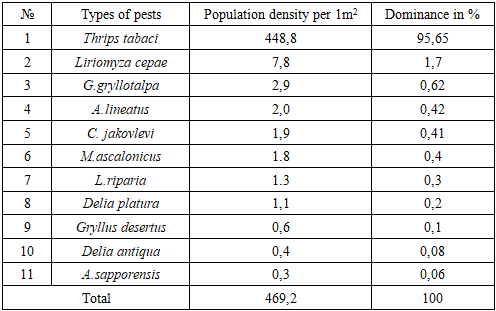
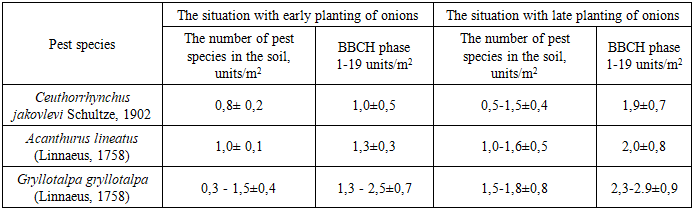
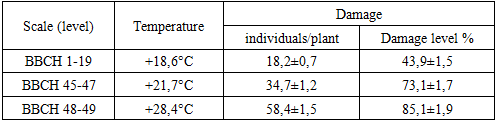
In the territory of northwestern Uzbekistan, 63 species of phytophages belonging to 8 orders, 29 families and 49 genera were identified. It was established that 5 species of phytophages are oligophages, 19 species are broadly polyphagous, 12 species are narrowly polyphagous. When determining the population density and dominance of the pest, it was noted that the species Thrips tabaci has the highest population density and dominance, the density of thrips per 1 m2 of area was 448.8, which is 95.65% higher than the dominance of other species. It was proven that the most widespread after Thrips tabaci were Liriomyza cerae (1.7%), Gryllotalpa gryllotalpa (0.62%), Agriotes lineatus (0.42%) and Ceuthorrhynchus jacovlevii (0.41%). The harmfulness of 7 species of phytophages living at the initial stage of onion leaf development (BBCH scale 1-19) was studied. Late planting of onions leads to an increase in the number of pests and a decrease in yield. Their number in crops increases and damage by phytophages increases.
Keywords: Onion pests, Exhauster, Light trap entomological net, BBCH, Dominance, Phase, Monophages, Poliphages, Oligophagous
Cite this paper: Abdullaev Ikram, Jumanazarov Hasanboy, Abduraxmanov Dilmurod, Iskandarov Abdulla, Aminov Muxammad, Sultanbaeva Jamila, Ecological Characteristics of Phytophagous Plants of the Onion Agrocenosis of Uzbekistan, International Journal of Genetic Engineering, Vol. 13 No. 8, 2025, pp. 152-158. doi: 10.5923/j.ijge.20251308.02.
Article Outline
1. Introduction
- Onion (Allium cepa L.) is one of the most important bulb vegetables cultivated in India and many other parts of the world. India ranks first in cultivation area and second in production [23], with approximately 1.3 million hectares under cultivation and an annual production of 22.40 million tonnes [9,29]. In Uzbekistan, winter onions and garlic were planted on a total area of 88.2 thousand hectares and in the Republic of Karakalpakstan and the Khorezm region, onions - on 16,023 hectares, garlic onions - on 1,281 hectares [14]. Onion is attacked by number of insect pests including thrips, maggots, cut worms, leaf miner, aphids, beetles, earwigs and mites at different stages of crop growth, including seedling, bulbing and blooming. [24,31]. In addition to direct feeding damage, certain insect pests, such as thrips (Thrips tabaci Lindeman) and aphids (Myzus persicae (Sulzer) and Aphis craccivora C.L.Koch), acts as vector for devastating viral diseases, including iris yellow spot virus [31], and onion yellow dwarf virus [24]. Furthermore, some insect pests cause damage to harvested bulbs during storage, reducing both quality and export potential. As onion is a major commodity for domestic consumption, export, and even import to meet national demand, accurate knowledge of its insect pests is essential for effective pest management planning. Therefore, an updated list of insect pests infesting onion in the field and storage is crucial for developing preventive and sustainable integrated pest management (IPM) strategies. This report presents a comprehensive list of onion insect pests [14].
2. Material and Methods
2.1. Study Area
- The Republic of Uzbekistan is located between the Amudarya and Syrdarya Rivers, and has a total area of 448,900 km2. The country extends approximately 1,425 km from east to west and 930 km from north to south [3,4,20,32]. The Northeast biogeographic region of Uzbekistan lies between the western part of the Tien Shan Mountains and the Syrdarya River [1,13,33]. Situated at the junction of several Central Asian biogeographical regions, Uzbekistan possesses remarkable richness in both flora and fauna. This diversity reflects the country’s wide range of natural conditions, encompassing vast plains with various desert types, mountain steppes, forests, alpine meadows, tugai thickets, and aquatic ecosystems [10,11,21]. Tugai and flood-plain ecosystems persist as small areas along the Amudarya, Syrdarya, Zarafshan, Chirchik, and Akhangaran rivers, though their extent is shrinking due to agricultural expansion and domestic use by local communities. Foothill plains and adyrs are located at the base of the western Tien Shan and Pamir-Alai ranges. These landscapes experience significant anthropogenic pressure from agriculture. Degraded low mountains and escarpments of the Ustyurt Plateau, situated within desert zones, harbor many rare species but are also under pressure from livestock grazing and mining activities (e.g., building stone, gypsum raw material). Alpine meadows, located 2,700 m above sea level. They are subject to significant pressure from animal husbandry. Uzbekistan has a low forest cover, with only about 7% of its land area under forests; nevertheless, forest ecosystems play an important role in both the economy and environmental protection [2,12,27]. Agriculture is a key sector of the national economy, and the country’s well-being and sustainable development depend greatly on the condition of its natural resources. More than 90% of crops are grown on irrigated land. Most natural ecosystems in Uzbekistan are highly vulnerable due to the arid climate, and their resistance to external pressures is low. Consequently, any anthropogenic activity serves as an additional driver of ecosystem degradation. The distribution of species in Uzbekistan is generally described according to four major biogeographic regions: (A) Ustyurt Plateau, (B) Kyzylkum Desert, (C) Pomir-Alai, and (D) Tien Shan. These regions are illustrated in Figure 1.
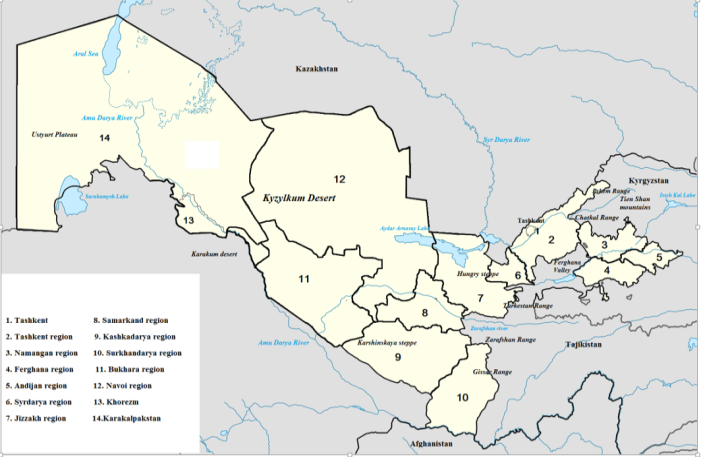 | Figure 1. Map Uzbekistan and location of North-western region of Uzbekistan |
2.2. Methods
- Studies on the species composition, as well as the morphological and ecological characteristics of onion phytophages inhabiting both soil and aerial parts of the plant, were conducted during 2023–2025. The research assessed their occurrence, mass reproduction, population density, and damage levels. Field surveys were carried out in onion plantations of the following farms: “Ogabek” and “Odamboy Hursand and Sayilkhon” in Qoshkopir District; “Beruniy Elita” in Khiva District; “Farrukh” in Urgench District; “Jumanov Anvar” in Beruniy District; “Alijon” in Ellikkal’a District; and “G‘alabali Asadbek” in Tortkul District. Additional observations were conducted in onion fields belonging to the experimental base of the Khorezm Mamun Academy (Fig. 2).
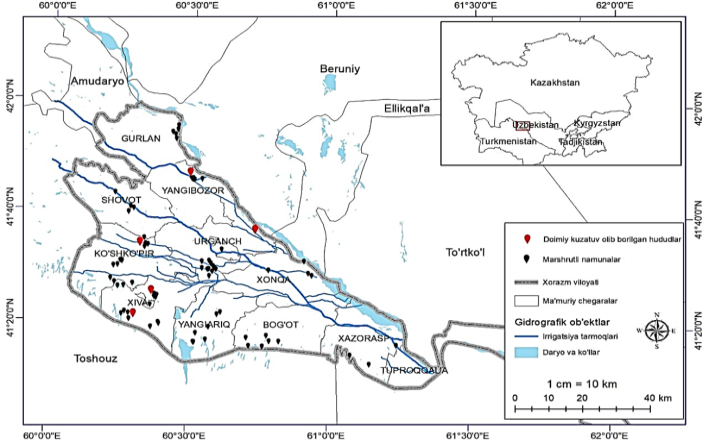 | Figure 2. Map of the areas where the research was conducted |
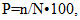 P - is the proportion of infested plants in the population (in %), n is the number of plants in the sample infested by the pest, and N is the total number of plants in the sample. As described above, survey materials were collected from onion farms located in the Ellikkal’a, Beruniy, and Tortkul districts of the Republic of Karakalpakstan, as well as from various districts of the Khorezm region. During the course of the study, a total of 6,478 insects were visually recorded, of which approximately 3,000 were used as primary research specimens; the majority of the remaining insects were released back into the wild. Insect collection was conducted following standard entomological procedures [18,25]. The capture of insects employed traditional methods, including the use of entomological sweep nets, aspirators, and insect traps (Fig. 3).
P - is the proportion of infested plants in the population (in %), n is the number of plants in the sample infested by the pest, and N is the total number of plants in the sample. As described above, survey materials were collected from onion farms located in the Ellikkal’a, Beruniy, and Tortkul districts of the Republic of Karakalpakstan, as well as from various districts of the Khorezm region. During the course of the study, a total of 6,478 insects were visually recorded, of which approximately 3,000 were used as primary research specimens; the majority of the remaining insects were released back into the wild. Insect collection was conducted following standard entomological procedures [18,25]. The capture of insects employed traditional methods, including the use of entomological sweep nets, aspirators, and insect traps (Fig. 3).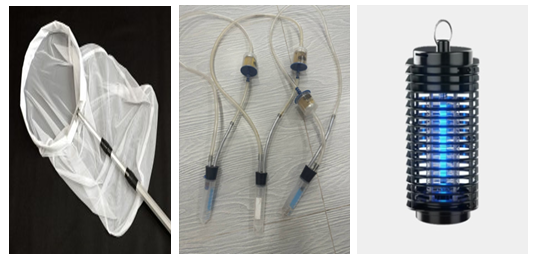 | Figure 3. Equipment used for collecting insects (A. Entomological net, В. Exhauster, С. Light trap) |
3. Results and Discussion
- Depending on the trophic characteristics of the plant species that the pests damage, they can be divided into three groups: monophages, feeding on only one plant; oligophages, damaging plants belonging to one family, i.e. the Rhizophagidae family; and poliphages, damaging plants belonging to several families. In our studies, the phytophagous pests identified were 2 species belonging to the monophagous group (Agriotes mancus, Suillia lurida), or 3.2% of the total species, and 14 species of oligophagous pests (Delia antiqua, Liriomyza cerae, Hydrellia griseola, Fannia canicularis, Fannia scalaris, Fannia leucosticte, Fannia manicata, Eumerus strigatus, Rhoralosirhum maidis, Acroleria sarrorensis, Acroleria asiatica, Mamestra brassicae, Pieris oligophagous, Ceutorrhynchus rare-wide oligophagous, Schizarchis ryri-tor oligophagous) or 22.2% of all pests were recorded, roliphagous 47 species were recorded, accounting for 74.6% of the total species, of which 3 species were broad roliphagous (Arhis gossyri, Myzus rersicae), 44 species were roliphagous (Ceuthorhynchus jacovlevii, Agriotes lineatus, Lasioderma serricorne, Melolontha melolontha, Melolontha hirrocastani, Melolontha furicicauda, Rentodon bisrinosus, Euborellia annulires, Delia rlatura, Drosorhila busckii, Labidura riraria, Muscina assimilis, Muscina stabulans, Myzus ascalonicus, Aulacorthum solani, Bemisia tabaci, Emroasca decirines, Macrosteles quadrilineatus, Nezara viridula, Carrorhilus obsoletus, Carrocoris fuscirinus, Dolycoris baccarum, Solenorsis sr, Srodothera exigua, Srodothera litura, Agrotis irsilon, Helicoverra armigera, Heliothis nubigera, Chrysodeixis chalcites, Chrysodeixis eriosoma, Noctua rronuba, Euxoa nigricans, Euxoa tritici, Hydraecia micacea, Trichorlusia ni, Hestia c-nigrum, Dolichovesrula sylvestris, Cryrtoblabes bistriga, Erhestia kuehniella, Loxostege sticticalis, Gryllotra gryllotalra, Gryllus desertus, Heteracris rterosticha, Thrirs tabaci) (Fig. 4).
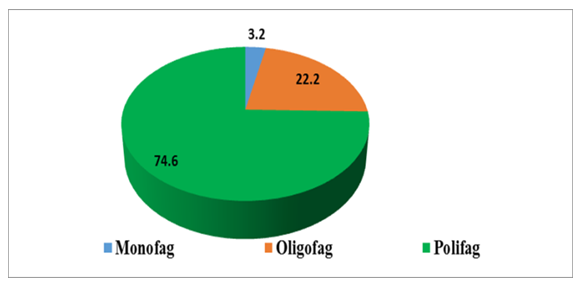 | Figure 4. Trophic groups of pests |
|
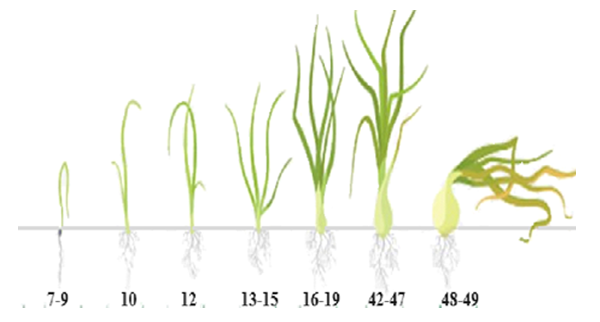 | Figure 5. Damage to onions by phytophagous insects in the Khorezm oasis (according to the BBCH classification scale) |
|
|
4. Conclusions
- The activity of pest insects was studied in the territory of northwestern Uzbekistan, and 63 species of phytophagous insects belonging to 8 orders, 29 families, and 49 genera were identified. Based on the trophic characteristics of pests damaging plant species, 5 species of phytophagous pests are oligophagous, 19 species are broad-polyphagous, and 12 species are narrow-polyphagous. When determining the population density and dominance of the pest, it was noted that the species Thrips tabaci has the highest population density and dominance; the density of thrips per 1 m2 of area was 448.8, which is 95.65% higher than the dominance of other species. It was proven that the species that spread the most after Thrips tabaci were Liriomyza cerae (1.7%), Gryllotalpa gryllotalpa (0.62%), Agriotes lineatus (0.42%), and Ceuthorrhynchus jacovlevii (0.41%). The harmfulness of 7 species of phytophagous insects living in the initial stage of development of onion plant leaves (BBCH scale 1-19) was studied. According to our research, late planting of onions can lead to an increase in pests and a decrease in yield. Their numbers in crops increase and damage by phytophagous insects increases.
ACKNOWLEDGMENTS
- We would like to express our gratitude to the Agency for Innovative Development under the Ministry of Higher Education, Science and Innovation of the Republic of Uzbekistan for funding this study under the project AL-19-562205691 Subject "Creation of agrotechnology for the combined protection of onion and garlic onion crops against pests".
CONFLICT OF INTERESTS
- The authors declare that there is no conflict of interest regarding the publication of this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML