A. O. Abdukarimov1, N. А. Ablakulova2, Y. B. Saidkulova3, Kh. Kh. Kushiev4
1PhD Student, Scientific Research Institute of Agrobiotechnologies and Biochemistry of Gulistan State University, Gulistan, Uzbekistan
2Senior Research Fellow, Scientific Research Institute of Agrobiotechnologies and Biochemistry of Gulistan State University, Gulistan, Uzbekistan
3Master’s Student, Scientific Research Institute of Agrobiotechnologies and Biochemistry of Gulistan State University, Gulistan, Uzbekistan
4Director, Scientific Research Institute of Agrobiotechnologies and Biochemistry of Gulistan State University, Gulistan, Uzbekistan
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This scientific research study investigated the effect of glycyrrhizic acid (GA) isolated from the roots of the liquorice plant (Glycyrrhiza glabra L.) and its complexes with certain microelements and phytohormones (IAA–indole 3–acetic acid; IBA–indole butyric acid; NAA–naphthyl acetic acid) on the most important morphogenetic and molecular–genetic traits of wheat and cotton seeds, such as germination, root system and stem development, chlorophyll grains and carotenoids. The results of this scientific study indicate high prospects for the use of glycyrrhizic acid complexes in agricultural practice to increase the resistance of plants to external stress factors and their productivity, as well as to optimize the process of growing environmentally friendly products. The results obtained indicate high prospects for the use of glycyrrhizic acid complexes in agricultural practice to increase the resistance of plants to external stress factors and their productivity, as well as to optimize the process of growing ecologically safe products.
Keywords:
Glycyrrhizic acid, Cotton, Complexes, Influence, Genetic traits, Plants, Phytohormones, Supramolecular complexes, Wheat, Germination rate, Chlorophyll, Carotenoid, Energy of germination
Cite this paper: A. O. Abdukarimov, N. А. Ablakulova, Y. B. Saidkulova, Kh. Kh. Kushiev, The Influence of Glycyrrhizic Acid Complexes on Genetic Traits of Plants, International Journal of Genetic Engineering, Vol. 13 No. 1, 2025, pp. 1-8. doi: 10.5923/j.ijge.20251301.01.
1. Introduction
In the field of agriculture, many types of chemical preparations (stimulants, herbicides, retardants, etc.) and large quantities of chemical substances are used to increase the yield of plants. However, the influences of most of the chemical preparations used on the morphogenetic traits and molecular genetic parameters of plants are unclear. This requires studying the growth and development of plants, as well as the quality and ecological safety of the product being grown [1-4]. Accordingly, the main goal in finding a solution to the existing problem in this field is to identify new physiologically active substances that regulate plant growth and development and have biostimulatory properties in connection with increasing plant productivity. In addition, nowadays, special attention is paid to protecting plants from external stress factors, reducing the use of synthetic chemical compounds, and using environmentally safe compounds based on natural substances, in connection with obtaining high–quality harvest. The complexes of phytohormones with steroidal natural physiologically active substances and stimulatory properties, isolated from natural sources, are used to protect plants from fungal and bacterial diseases [1-4]. This scientific work also notes some results on increasing the level of resistance of agricultural crops to external stress factors with the help of bioregulators, as well as the most important biological indicators at the stages of growth and development [5,6].Clarifying the importance of phytohormones in the formation of resistance mechanisms in plant organisms under the influence of various stress factors, and developing optimization technologies, methods, and approaches is an actual issue from a theoretical/practical point of view [7].In this scientific work, studies were conducted to study the influence of glycyrrhizic acid compounds on certain morphogenetic and molecular–genetic parameters of cotton (Gossypium barbadense L.) and wheat (Tritcum aestivum L) seeds during germination, growth, and development.
2. Materials and Methods
The studies investigated the influence of glycyrrhizic acid (Figure 1) isolated from the roots of the liquorice plant (Glycyrrhiza glabra L.) and its GA:PHs supramolecular complexes synthesized with certain microelements and Phs (IAA, IBA, NAA) [8-10] on the germination, growth, and development of wheat and cotton seeds.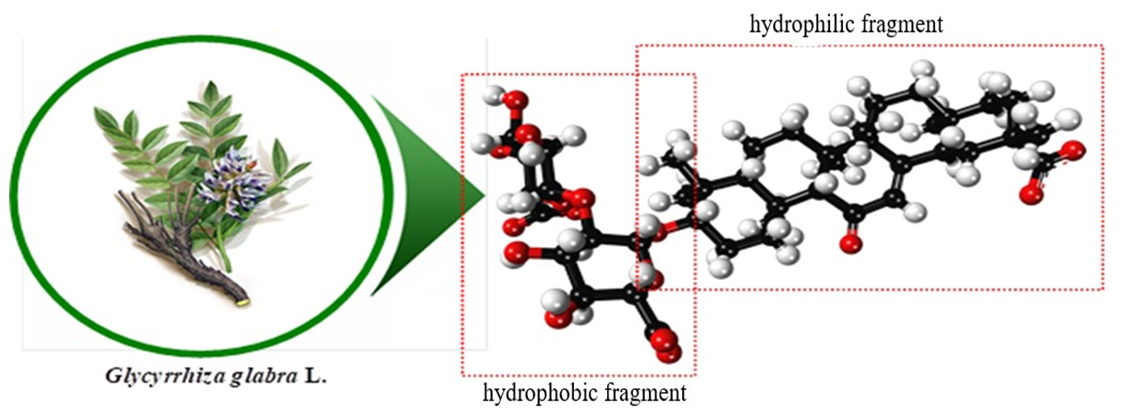 | Figure 1. The chemical structural formula of glycyrrhizic acid (Empirical formula–C42H62O16; 20β–carboxy–11–oxo–30–norolean–12–en–3β–yl–2–О–β–D–glucopyranuronosyl–α–D–glucopyranosiduronic acid) [Shlotgauer, 2013, p. 553–556; Yakovshin, 2018, p. 10–19] |
The seeds of cotton (Gossypium barbadense L.) Ravnaq–1 and wheat (Triticum aestivum) Dostlik varieties were analyzed based on the study of germination indicators under laboratory conditions. During the research, cotton and wheat seeds were sterilized in NaClO (2%) solution for 5 minutes [11] or KMnO4 (1%) solution or in ethanol solution (70%) for 2 minutes [12], then washed in a stream of distilled water and grew in a Petri dish [13]. The Petri dish was sterilized using ethanol solution (70%). In each dish, 50 cotton and 100 wheat seeds were cut to a size equal to the diameter of the Petri dish and placed on filter paper soaked in distilled water (10 ml) [6,14].The experiments used filter papers “Vаtmаn №1” (“Sigma–Aldrich”; Germany) and D=110 (“Хimrеаktivkоmplеkt”; Russia).Germination of plant seeds was carried out for 10 days (240 hours) in a dark state, in a thermostat at a temperature of +22°C. After 24 hours, the germination process began in the seeds (Figure 2). | Figure 2. The condition of studying the level of germination of cotton (A) and wheat (B) seeds treated with GA:PHs complexes in the laboratory |
During the germination process, germination energy was calculated on the 3 days (72 hours) and the germination rate of the seeds was recorded on the 10 days [14].A seed was considered germinated if it produced a root longer than half its length [14].Germination energy expresses the percentage of grains that have germinated at a standard rate over a given period of time (3 days) compared to the total number of grains [15,16].Germination rate–generally expressed as the percentage of grains that germinated at a standard rate relative to the number of seeds used in an experiment [16].In the experiments, the germination rate (GA) was calculated using the following formula [17]: Here D represents the number of experimental days in which the calculation was carried out. GA: Study of the effect of PHs supramolecular complexes on the content of chlorophylls and carotenoids in plants.The effect of the cotton variety “Ravnaq 1” on the content of chlorophylls and carotenoids.To study the effect of GA:PHs complexes on the amount of chlorophyll in plant seedlings, 50 mg of cotton leaves were placed in a test tube. Each leaf sample was homogenized in 5 ml of 95% ethyl alcohol solution and centrifuged for 30 minutes at 4500 rpm. The amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the resulting liquid were determined by light absorption at 664, 649 and 470 nm (HACH DR 3900 spectrophotometer). Based on this indicator, the amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the leaves of the cotton plant were calculated using the Lichtentaler equation [8].Chlorophyll “a” [mg/g]=13,36*A664–5,19*A649Chlorophyll “b” [mg/g]=27,43*A649–8,12*A664Carotenoid [mg/g]=(1000 A470–2,13*Xlo “a”–97,63 Xlo “b”)/209F(mg/g)=(V*C)/PWhere: F–amount of pigment in the plant leaf (mg/gr); V–volume of liquid (ml); C–pigment concentration (mg/l); P–mass of plant tissue.Statistical analysis of the experimentally obtained total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content of plants was performed in EXCEL 2016, using the Stat View program using ANOVA.Mathematical and statistical processing of the obtained results. The experimental results were mathematically and statistically processed using the special software package OriginPro v. 8.5 SR1 (EULA, USA) according to standard biometric methods [18-21].
Here D represents the number of experimental days in which the calculation was carried out. GA: Study of the effect of PHs supramolecular complexes on the content of chlorophylls and carotenoids in plants.The effect of the cotton variety “Ravnaq 1” on the content of chlorophylls and carotenoids.To study the effect of GA:PHs complexes on the amount of chlorophyll in plant seedlings, 50 mg of cotton leaves were placed in a test tube. Each leaf sample was homogenized in 5 ml of 95% ethyl alcohol solution and centrifuged for 30 minutes at 4500 rpm. The amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the resulting liquid were determined by light absorption at 664, 649 and 470 nm (HACH DR 3900 spectrophotometer). Based on this indicator, the amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the leaves of the cotton plant were calculated using the Lichtentaler equation [8].Chlorophyll “a” [mg/g]=13,36*A664–5,19*A649Chlorophyll “b” [mg/g]=27,43*A649–8,12*A664Carotenoid [mg/g]=(1000 A470–2,13*Xlo “a”–97,63 Xlo “b”)/209F(mg/g)=(V*C)/PWhere: F–amount of pigment in the plant leaf (mg/gr); V–volume of liquid (ml); C–pigment concentration (mg/l); P–mass of plant tissue.Statistical analysis of the experimentally obtained total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content of plants was performed in EXCEL 2016, using the Stat View program using ANOVA.Mathematical and statistical processing of the obtained results. The experimental results were mathematically and statistically processed using the special software package OriginPro v. 8.5 SR1 (EULA, USA) according to standard biometric methods [18-21].
3. Results and Discussions
In the experiments, it was found that in the control variant, the average weight of 1 cotton seed was 48.2±1.3 mg, and the average weight of 1 wheat seed was 41.7±2.4 mg. After the water absorption phase (72 hours), the cotton seed increased by 54.6±2.7% and the wheat seed by 47.5±3.5% compared to the control, i.e., an average of 63.92±2.4 and 72±2.1 mg, respectively. It was noted that the intensity of water absorption will be high for the first 16–72 hours (1–3 days).It was found that under the influence of GA:PHs (IAA, IBA, NAA) supramolecular complexes (100 μM), the intensity and amount of water absorption dynamics of cotton and wheat seeds during germination under laboratory conditions increased compared to the control.The results obtained are consistent with the available literature data [6]. During the germination process, cotton seeds enter the germination phase after absorbing ~45–50% of their dry weight [6]. Studies have shown that the water absorption phase of cotton seeds lasts ~2–6 hours, and in the next phase (~6–16 hours), water reaches the endosperm, during which the activation of enzymatic reactions occurs [22].During the process of seed germination, PHs perform the most important physiological function as essential endogenous regulators [6].Studies have shown that GA optimize the permeability of biological membranes [23-25].The results obtained can be explained by the optimization of the permeability properties of biological membranes under the influence of supramolecular complexes of GA:PHs (IAA, IBA, NAA) (100 μM).In the course of the research, in order to draw clear conclusions about the effect of supramolecular complexes of GA:PHs (IAA, IBA, NAA) on the intensity of water absorption dynamics of seeds, cotton seeds were treated with complexes and planted in soil beds. The results obtained during the research were consistent with laboratory results, and corresponding changes were also detected in the amount of chlorophyll and carotenoids in plant seedlings (Table 1).Table 1. Effect of supramolecular complexes of GA:PHs (IAA, IBA) on the content of chlorophylls and carotenoids in the cotton variety “Ravnaq 1” under laboratory conditions
 |
| |
|
Table 2. Effect of GA:PHs (IAA, IBA) supramolecular complexes on the content of chlorophylls and carotenoids in wheat under laboratory conditions
 |
| |
|
In molecular genetic research, quantitative and qualitative indicators are determined based on DNA. Accordingly, during our research, we isolated DNA from plant tissues.For DNA extraction, leaves were collected from plants treated with supramolecular complexes consisting of GA and PHs and from control plants as biological materials. Genomic DNA was extracted from plant leaf tissues using the STAB method.The isolated DNA molecules were electrophoresed on an ethidium bromide–stained agarose gel for 1 hour at 80 V. A DNA marker with an initial step of 50 bp was used to compare the weights of the DNA samples on the gel (Figure 3). | Figure 3. Visual representation of DNA samples extracted using the STAB method on a 0.9% agarose gel |
The DNA samples showed bright and pure bands in the agarose gel image. These indicators indicate that the DNA samples were of high purity and free from protein and organic matter contamination. High–purity DNA molecules allow for more efficient subsequent steps in the research.DNA quantitative and qualitative parameters were studied using a Nanodrop spectrophotometer (Thermofisher). TE buffer was used as the measurement. DNA samples were taken and measured in 2 μl (Table 3).Table 3. The effect of GA:PHs (IAA, IBA) supramolecular complexes on the quantitative and qualitative parameters of the cotton variety “Ravnaq 1” in laboratory conditions
 |
| |
|
For PCR and subsequent studies, the optimal DNA concentration is usually above 50 ng/μl, i.e. purity values of A260/280 are in the range of 1.8-2, and A260/A230 values are 2.0–2.2.The fact that the quantitative and qualitative characteristics of the extracted DNA samples are compatible with many PCR markers allows for the use of a wider range of markers for research purposes.Accordingly, during the studies conducted, we investigated the effect of GA:PHs supramolecular complexes and trace element salts of GA on the variability of morphogenetic traits in wheat during the growth and development stages.In this regard, based on the results of the action of glycyrrhizinic acid salts, corresponding positive changes were detected in the morphological characteristics of plants. Based on the detected changes and their results, it can be noted that glycyrrhizinic acid salts increase the activity of the most important expression genes that control the ontogenetic development of the plant. This led to the emergence of corresponding positive changes in the morphological characteristics of the plant, increasing its yield indicators and resistance to external stress factors.One of the ways to study the exogenous effects of physiologically active compounds is to use molecular markers. Molecular markers are based on RNA polymorphisms and are a highly valuable and effective tool for classification. In this study, randomly amplified RNA fragments (RAPD), amplification of simple sequence repeats (ISSR), and amplification of regulatory fragments of microRNA (miRNA) molecules were used as molecular markers based on RNA polymorphisms.In our research, we soaked wheat seeds in solutions of various concentrations of cobalt diglycyrrhizinate (1%, 0.1%, 0.01% and 0.001%) for 2 hours and then placed them in a nutrient medium. We analyzed the germination rate of wheat seeds after 2 days and the effect of cobalt diglycyrrhizinate on fungi in vitro after 5 days.According to the results of the analysis, RNA polymorphism was observed in the roots and leaves of the control wheat seedlings. RNA–RAPD fragment profiles were recorded in almost every concentration sample (Figure 4).  | Figure 4. RAPD–polymorphism analysis of 5–day–old wheat seedlings treated with cobalt diglycyrrhizinate solutions: M–marker, C–control wheat seedlings. The similarity of DNA profiles with control samples is noted |
In this case, the presence of the RAPD direction prevents the dominant trait from opposing the recessive trait. Thus, this is consistent with the conclusion [26,27] that heterozygous and homozygous dominant individuals are not distinguished by RAPD markers.Comparative analysis of RAPD profiles of individual, i.e., separately obtained samples, revealed that ISSR fragments are almost monomorphic (Figure 5). No variability was observed in the polymorphism of DNA isolated from wheat leaf tissue, similar to the differences in the ISSR fragment profile in root tissue. The profile of the control sample of wheat genotypes and the lowest (0.001%) elicitor concentration of the sample is unique compared to the one at the highest concentration. This indicates that the DNA micro–site polymorphisms are affected in a manner that is based on.  | Figure 5. Polymorphism analysis using ISSR markers in 5–day–old wheat seedlings treated with cobalt diglycyrrhizinate solution: M–marker, C–control plant |
A conserved type of miRNA family was selected to test the effect of cobalt diglycyrrhizinate on wheat seedlings. The miR168 family is considered a marker of plant response to stress factors [28]. Our studies revealed that the miR395 locus amplification index in wheat tissue samples treated with cobalt diglycyrrhizinate solution increased several times compared to the control (Figure 6).  | Figure 6. Quantitative indices of 18 miRNA loci amplified in leaf and root tissues of experimental and control samples of wheat seedlings |
It was found that this indicator is higher in root tissues than in leaf tissues of wheat.Analysis of miR168 parameters under the influence of cobalt diglycyrrhizinate shows that the average effect coefficient of cobalt diglycyrrhizinate varied from 27.63 to 28.69, with a standard deviation of 0.24 to 0.66. The miR395 parameter was much lower, with an effect coefficient of 34.86 to 35.52, with a standard deviation of 0.35 to 1.52 (Figure 7). | Figure 7. miR395 expression in wheat leaves treated with cobalt diglycerides |
When both miRNAs were analyzed, their abundance was reduced in the presence of 1% cobalt diglycyrrhizinate (11.76% for miR168 and 1.5% for miR395). When compared with the effects of 0.1% and 0.01% cobalt diglycyrrhizinate solutions, it was found that miR168 increased by 1.87% and miR395 by 9.52%.
4. Conclusions
The effective effect of supramolecular complexes and salts of glycyrrhizic acid was distinguished by the longer survival of plant leaves compared to control samples.Thus, increasing the yield and quality of agricultural crops and ensuring food security are considered one of the strategic priorities, and the prospects for using environmentally safe endogenous phytoregulators that have the ability to optimize the germination and development indicators of agricultural crop seeds in solving this problem are highly appreciated.It has been found that the use of endogenous phytoregulators in agricultural practice makes it possible to increase growth and development, productivity, as well as resistance to the effects of various phytopathogens and stress factors in the plant organism through the stimulation of complex biochemical/physiological processes.
References
| [1] | Bayshanova A.E., Kedelbaev B.Sh. (2016) Problems of soil degradation. Analysis of the current state of fertility of irrigated soils of the Republic of Kazakhstan // Scientific review. Biological sciences. – 2016. – No.2. – p. 5–13. |
| [2] | Belozerova A.A., Bome N.A. (2014) Study of the response of spring wheat to salinity by the variability of morphometric parameters of seedlings // Fundamental research. –2014. – No.12–2. – p. 300–306. |
| [3] | Koshiev Kh.Kh. (2011) Controlling the influence of biotic and abiotic factors on the growth and development of wheat using physiologically active substances // Abstract of the dissertation written for the degree of Doctor of Philosophy (02.00.10–Bioorganic chemistry). – Gulistan, 2011. – p. 9–231. |
| [4] | Djuraev T., Kushiev Kh.Kh. and Gafurov M.B. (2018) Stimulating Properties of Components Glycyrrhizic Acid in Growth and Development of Wheat (Triticum aestivum) // J. Biol. Chem. Research. 2018. – Vol.35. – No. 2., – p. 323–310. |
| [5] | Isaev R.F., Grishina L.I. (2007) Efficiency of application of biological and anti–stress preparations on spring wheat crops // Agrochemical Bulletin. – 2007. – No.6. – p. 32–33. |
| [6] | Tagaeva H.E. (2019) Growth–regulating activity of glycerol derivatives on the germination of soft wheat seeds // Dissertation for the PhD. (03.01.05–Plant Physiology and Biochemistry). – Dushanbe, 2019. – p. 3–20. |
| [7] | Abramova A.S. (2016) The influence of biological preparations on the yield structure of spring soft wheat under stress conditions // International School Scientific Bulletin. – 2016. – No.4. – p. 9–11. |
| [8] | Kondratenko R.M., Baltina L.A., Mustafina S.R. et al. (2001) Method synthesis of crystalline glycyrrhizic acid from industrial Glycyrram. Immunomo dulating properties // Chem. Pharm. Journal. – 2001. – V.35. – p. 38–42. |
| [9] | Astafeva O.V., Suxenko L.T., Yegorov M.A. (2013) Antimicrobial activity of isolated biologically active substances and root extract of Glycyrrhiza glabra L. // Chemistry of plant raw materials. – 2013. – No.3. – p. 261–263. |
| [10] | Shlotgauer A.A. (2013) Study of the interaction of atorvastatin with the triterpene glycoside glycyrrhizic acid by the NMR relaxation method in solutions // Fundamental research. – 2013. – No.10–3. – p. 553–556. |
| [11] | Stanojevic D., Dordevic S., Simic B., Radan Z. (2014) Wheat seeds (Triticum aestivum L.) growth promotion by bacteria auxin, in vitro // In: Proceedings of the 49th Croatian and 9th International Symposium on Agriculture. – Dubrovnik (Hrvatska). – 2014. – p. 97–101. |
| [12] | Bardina L.E. (2019) Chemical growth regulators and their application: Guidelines for laboratory work. |
| [13] | Alenkina S.A., Nikitina V.E. (2016) The effect of azospirillum lectins on the activity of proteolytic enzymes and their inhibitors in the roots of wheat sprouts // Bulletin of the Samara Scientific Center of the Russian Academy of Sciences. – 2016. – Vol.18. – No.1. – p. 5–11. |
| [14] | Userbaeva B.A., Bozshataeva G.T., Ospanova G.S., Turabaeva G.K. (2015) The influence of different salt concentrations on the germination of grain crop seeds // International Journal of Experimental Education. – 2015. – No.3–1. – p. 65–67. |
| [15] | Davidyants E.S. (2011) Effect of triterpene glycosides on the activity of α– and β–amylases and the content of total protein in wheat sprouts // Applied Biochemistry and Microbiology. – 2011. – Vol.47. – No.5., – p. 530–536. |
| [16] | Rubets V.S. (2016) Biological characteristics of triticale as a basis for improving the breeding process // Abstract of diss....Doctor of Biological Sciences. – Moscow, 2016. – p. 28–29. |
| [17] | Hassan A.A. (2015) Germination and growth of wheat plants (Triticum aestivum L.) under salt stress // Journal of Pharmaceutical, Chemical and Biological Sciences. – 2015. – Vol. 3 (3). – p. 416–420. |
| [18] | Polevoy V.V., Chirkova T.V., Lutova L.A. (2001) Workshop on plant growth and stability // St. Petersburg: Publishing house of St. Petersburg University, 2001. – p. 35–212. |
| [19] | Chachar Q.I., Solangi A.G., Verhoef A. (2008) Influence of sodium chloride on seed germination and seedling root growth of cotton (Gossypium hirsutum L.) // Pak. J. Bot. – 2008. – V. 40(1). – p. 183–197. |
| [20] | Shohani F., Mehrabi A.A., Khavarinegad R.A., Safari Z., Kian S. (2014) The effect of gibberellic acid (GA3) on seed germination and early growth of lentil seedlings under salinity stress // Middle–East Journal of Scientific Research. – 2014. – Vol. 19 (7). – p. 995–1000. |
| [21] | Dospexov B.A. (2014) Methodology of field experiment (with the basics of statistical processing of research results) // Moscow. – Publishing house “Agroproizdat”. – 2014. – p. 110–351. |
| [22] | Рогожина Т.В., Рогожин В.В. (2011) Физиолого–биохимические механизмы прорастания зерновок пшеницы // Вестник АГАУ. – 2011. – о№8. – С.17–21. |
| [23] | Dushkin A.V., Metelyova E.S., Chistyachenko Yu.S., Khalikov S.S. (2013) Mechanochemical preparation and properties of solid dispersions forming water–soluble supramolecular systems // Fundamental research. – 2013. – No.1–3. – p. 741–749. |
| [24] | Insightful and penetrable // [Electronic resource]. http://www.sbras.info/articles/science/pronitsatelnye-i-pronitsaemye. |
| [25] | How glycyrrhizic acid improves the permeability of cell membranes // [Electronic resource]. https://scientificrussia.ru/articles/kak-glitsirrizinovaya-kislota-uluchshaet-pronitsaemost-kletochnyh- membran. |
| [26] | V.I. Tainskiy, Peculiarities of harmfulness of corn loose, root putrid and brown rust of spring wheat / I.P. Naumova, A.G. Gaponova, N.G. Bey–Bienko // Agricultural biology. – 2002, No. 3, – p. 104–108. |
| [27] | Katarína Ražná, Nodira Ablakulova, Jana Žiarovská, Matúš Kyseľ, Khabibjhan K. Kushiev, Maxmudjhan B. Gafurov, Ľudovít Cagáň (2020) Molecular characterization of the effect of plant–based elicitor using microRNAs markers in wheat genome // Biologia. – Volume 75, – p. 2403–2411. |
| [28] | N.V. Polyakova. The role of abscisic acid in lesion of barley with helminthsporium // VI International Conference on Growth regulators and development of plants in biotechnologies (June 26–28, 2001). – Moscow, 2001. – p. 56–57. |



 Here D represents the number of experimental days in which the calculation was carried out. GA: Study of the effect of PHs supramolecular complexes on the content of chlorophylls and carotenoids in plants.The effect of the cotton variety “Ravnaq 1” on the content of chlorophylls and carotenoids.To study the effect of GA:PHs complexes on the amount of chlorophyll in plant seedlings, 50 mg of cotton leaves were placed in a test tube. Each leaf sample was homogenized in 5 ml of 95% ethyl alcohol solution and centrifuged for 30 minutes at 4500 rpm. The amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the resulting liquid were determined by light absorption at 664, 649 and 470 nm (HACH DR 3900 spectrophotometer). Based on this indicator, the amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the leaves of the cotton plant were calculated using the Lichtentaler equation [8].Chlorophyll “a” [mg/g]=13,36*A664–5,19*A649Chlorophyll “b” [mg/g]=27,43*A649–8,12*A664Carotenoid [mg/g]=(1000 A470–2,13*Xlo “a”–97,63 Xlo “b”)/209F(mg/g)=(V*C)/PWhere: F–amount of pigment in the plant leaf (mg/gr); V–volume of liquid (ml); C–pigment concentration (mg/l); P–mass of plant tissue.Statistical analysis of the experimentally obtained total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content of plants was performed in EXCEL 2016, using the Stat View program using ANOVA.Mathematical and statistical processing of the obtained results. The experimental results were mathematically and statistically processed using the special software package OriginPro v. 8.5 SR1 (EULA, USA) according to standard biometric methods [18-21].
Here D represents the number of experimental days in which the calculation was carried out. GA: Study of the effect of PHs supramolecular complexes on the content of chlorophylls and carotenoids in plants.The effect of the cotton variety “Ravnaq 1” on the content of chlorophylls and carotenoids.To study the effect of GA:PHs complexes on the amount of chlorophyll in plant seedlings, 50 mg of cotton leaves were placed in a test tube. Each leaf sample was homogenized in 5 ml of 95% ethyl alcohol solution and centrifuged for 30 minutes at 4500 rpm. The amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the resulting liquid were determined by light absorption at 664, 649 and 470 nm (HACH DR 3900 spectrophotometer). Based on this indicator, the amounts of chlorophyll “a”, chlorophyll “b” and carotenoids in the leaves of the cotton plant were calculated using the Lichtentaler equation [8].Chlorophyll “a” [mg/g]=13,36*A664–5,19*A649Chlorophyll “b” [mg/g]=27,43*A649–8,12*A664Carotenoid [mg/g]=(1000 A470–2,13*Xlo “a”–97,63 Xlo “b”)/209F(mg/g)=(V*C)/PWhere: F–amount of pigment in the plant leaf (mg/gr); V–volume of liquid (ml); C–pigment concentration (mg/l); P–mass of plant tissue.Statistical analysis of the experimentally obtained total chlorophyll, chlorophyll a, chlorophyll b, and carotenoid content of plants was performed in EXCEL 2016, using the Stat View program using ANOVA.Mathematical and statistical processing of the obtained results. The experimental results were mathematically and statistically processed using the special software package OriginPro v. 8.5 SR1 (EULA, USA) according to standard biometric methods [18-21].



 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML



