Abdullaeva Kh1, Makhkamova D.2, Iskhakova Sh3
1Researcher Department of Soil, National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan
2Dsc., Associate Professor, Department of Soil, National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan
3PhD., Associate Professor, Department of Soil, National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan
Correspondence to: Abdullaeva Kh, Researcher Department of Soil, National University of Uzbekistan named after Mirzo Ulugbek, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents data on the types of gypsum of varying degrees, prevalent in the Zarbdor district of the Jizzakh region, and the number of individual microorganisms depending on the depth of their location. Among the studied groups of microorganisms, ammonifiers predominated, followed by oligonitrophils, nitrogenobakteres, and nitrifying microorganisms. It has been established that the influence of gypsum content on the number of physiological groups of microorganisms presented in the article varies. In terms of gypsum content, a decrease in the activity of microorganisms was observed in the direction of non-gypsum-low-medium-high soils.
Keywords:
Irrigated serozem-meadow soils, Gypsum, Salt and gypsum crystals, Gypsum index in the soil, Microorganism, Role of microorganisms, Bacteria
Cite this paper: Abdullaeva Kh, Makhkamova D., Iskhakova Sh, The Influence of Gypsum Content and Depth of Location on the Activities of Microorganisms in the Soils of the Studied Area, International Journal of Genetic Engineering, Vol. 12 No. 6, 2024, pp. 123-127. doi: 10.5923/j.ijge.20241206.11.
1. Introduction
Uzbekistan differs from other regions in its climate, geological and geomorphological structure. Therefore, it can be seen that soil types are divided into several types. One of them is gypsum soils. Although these soils began to form in the regions of the republic with some specific hydrological conditions, their impact on agriculture is significant.It is known that gypsum soils are found in small quantities in Syrdarya, Jizzakh, Navoi, and partially in Surkhandarya, Ferghana, and Kashkadarya. Gypsum, according to scientists, is formed by strong salinization. Currently, gypsum soils account for more than 383.2 thousand hectares of irrigated land in our republic, which is 10.3% of the total area of irrigated agricultural land. Of these, 5.1% of lands with varying degrees of gypsum are weakly gypsum-bearing, 3.5% are moderately gypsum-bearing, and 1.8% are strongly and very strongly gypsum-bearing soils. The specific groundwater of each massif is characterized by its proximity to the surface layer of the soil and its high mineralization.In these soils, the direction and intensity of processes occurring in the water-soil-plant system, as well as their quantitative indicators, undergo constant changes over time, subject to horizontal laws. Therefore, some soils are classified as difficult to develop [5].
2. Material and Methods
Gypsum soils are widespread not only in Uzbekistan, but also in Central Asia. In the first half of the 20th century, gypsum soils of Ustyurt began to be studied by Russian-speaking scientists, and their paleohydromorphic origin was determined.Heavily saline serozem-meadow soils, common in the southeastern part of Mirzachul, can be classified as difficult-to-reclaim soils. The mechanical composition of these soils was layered, and at a certain depth, a layer of heavily compacted gypsum was formed. (Syrdarya and Jizzakh regions). The presence of a sharply pronounced layer of soil, a solid gypsum layer, or a branched layer reduces water filtration. It will take several years to improve the reclamation status of these soils. V.A. Kovda (1946), V.V. Egorov (1954), A.T. Morozov, Yu.P. Lebedev (1954) and others explain the appearance of gypsum as salt solutions moving in chalk and tertiary rocks, alluvial and deluvial-proluvial deposits, in the foothill and alluvial plains of Transcaucasia and Central Asia, forming a layer of deposits in the form of gazh, arzyk, and shokh [1]. E. V. Lobova (1960) and I. Bobohodjaev (1960) explained the formation of gypsum in light sierozem soils of the desert zone and the Nuratau foothills through their research [2]. N.A. Buskov and A.M. Nosirov (1961) in their work "Soils of South-Western Kyzylkum" provided detailed information about the gypsum-sandy soils of Malikchul and Kyzylkum, which have a dry climate in Uzbekistan [3]. N.G. Minashina, N.G. Minashina, L.L. Shishov, G.L. Gavrilova, E.I. Pankova, I.A. Yamnova, A.U. Akhmedov, G.T. Parpiev, S.A. Abdullaev, L.A. Gafurova, and D.Yu. Makhkamova conducted research on the reclamation status, agrochemical properties, and biological activity of soils in the Jizzakh and Syrdarya regions [4]. Until recently, the origin of hydromorphic gypsum soils seemed easier to explain than that of automorphic soils. It was believed that the process of gypsum accumulation occurs due to the accumulation of calcium sulfate in groundwater. If we look at the evidence and facts collected now, it becomes clear that this process is somewhat complex. Despite the fact that the study of gypsum soils by a number of scientists continues to this day, there is still no consensus on the formation of gypsum, its influence on the physical and chemical properties of soils and cultivated plants [2]. Scientific research was conducted using the "Methods of Agrochemical, Agrophysical, and Microbiological Research in Cotton Regions" of the Uzbek Scientific Research Institute, "Guidelines for Chemical Analysis of Soils" by E.V. Arynushkin, as well as the "Methods of Soil Microbiology and Biochemistry" of the Research Institute of Soil Science and Agrochemistry and Soil Microorganisms by M. Zvyaginsev. The gypsum content of soils was determined according to the classification developed by E.I. Pankova. In laboratory conditions, gypsum (SO4) was displaced by an acidimetric method in a 0.2-normal hydrochloric acid (HCL) solution.
3. Results and Discussion
Gypsum soils (CaSO4*2H2O) have their own characteristics. It differs from other soil types in its main morphological features, general physical properties, chemical and reclamation properties. The development of gypsum soils is indeed a complex and time-consuming process. Examples of low productivity in such soils include several main factors: deterioration of water-physical properties, high salinity, compaction of the soil surface, suffusion phenomena, and low nutrient content [6]. If the gypsum content in the soil is less than 10%, it can be classified as non-gypsum soils. In this case, the presence of gypsum does not negatively affect plant growth. According to a more accurate definition, gypsum is actively involved in metabolism and is characterized as not gypsiferous in such soils. If the amount of gypsum accumulated in the soil layers exceeds 10%, then it is called gypsum soils, and the determination of the degree of gypsum accumulation of such soils is carried out based on the classification proposed by N.G. Minashina (1978) (Table 1).Table 1. Classification for Determining the Degree of Soil Gypsification
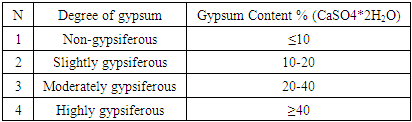 |
| |
|
Due to the fact that the gypsum layer in the soils of the studied area is mainly located at a depth of 70-10 cm, deeply gypsum-bearing soils are considered, and in some sections, it is observed that they are located on the surface and deeper. Depending on the depth of placement of the gypsum layer formed along the soil profile, it is divided into soil differences, which are interpreted differently in various sources (Table 2).Table 2. Depending on the depth at which the gypsum is located, starting from its upper boundary
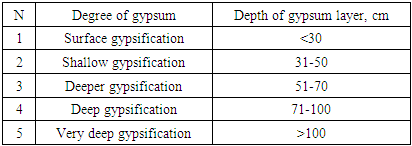 |
| |
|
According to laboratory analysis of soil samples taken from the study area, the amount of gypsum (CaSO4*2H2O) in the plow layer of non-gypsum soils (sections 4, 5, 6, 7, 8) was 3.49-1.84-7.78-8.28.1.28%, while in the subsoil layer it was 1.28-4.97-9.33-1.09%. In weakly gypsum-bearing soils (sections 1, 2, 3), the gypsum content in the plow layer of the soil horizon increases to 18.04-10.30-15.65%, in the middle parts of the profile to 12.38-18.21%. The highest gypsum content was observed in moderately gypsified soil sections (sections 8, 7, 9, 10) and amounted to 22.69-25.40% in the plow layer and 22.09-27.17-36.23% in the subsoil. An increase of 42.35-46.91-56.25% was observed in the highly gypsumized subsoil (sections 6, 9, 10) (Fig. 1).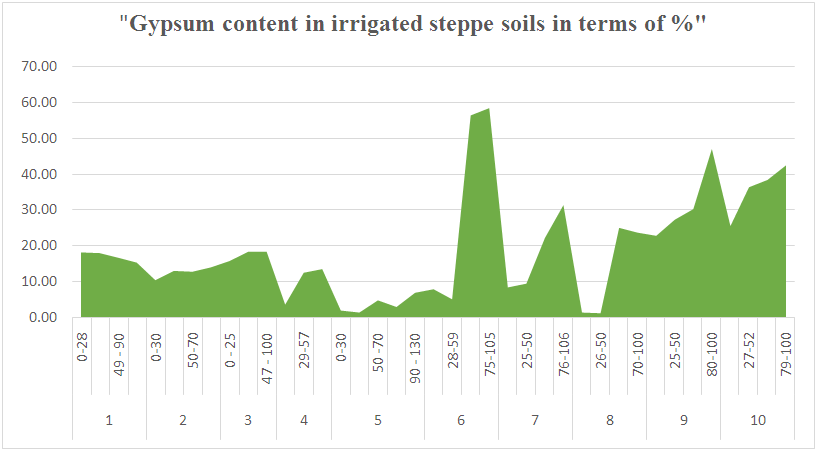 | Figure 1. The gypsum content of the region's soils, in % |
Adaptation of the root system of plants to soil conditions is one of the important ecological processes. The degree of soil gypsum formation, i.e., the high content of gypsum (CaSO4·2H2O) in its composition, significantly affects the normal growth and development of plants. This circumstance can lead to several negative processes. According to the provided data, the thickness of the gypsum layer in the soil profiles of the area is within the range of 40-60 cm, and this layer belongs to the group of gypsum-bearing soils.These soils are classified as gypsum-containing soils of medium thickness (40-100 cm) according to the gradation of the gypsum layer thickness. In the upper part of the profile: gypsum particles consist of fine flour-like (less than 0.1 mm) "spotted" crystals, while in the lower part, gypsum particles are found in the form of fine-grained (0.1-1.0 mm) and medium-grained (1.0-10.0 mm) crystals, which are in a scattered or alloy state. Gypsum (CaSO4·2H2O) can be found in the soil layer in various forms: crystals, granular masses, or other impurities. Although dry gypsum in the plow layer is dense and cohesive, the ability of its structure to easily decompose upon water saturation is due to the fact that it penetrates the crystalline structure of water and loosens it. Water molecules alter the structure of hydrated gypsum, transforming it into granular masses. Mechanical impacts (e.g. pumping or machining) cause gypsum to break into light pieces due to its brittleness. This property is important for soil composition and agricultural cultivation, as the physical condition of the soil affects plant growth and the water-air regime. In the lower layers of the soil profile, rod-shaped prismatic medium and coarse-crystalline gypsum crystals are located in the form of interspersed crystals, which retain their structure even when the soil is moistened between large cavities [7].The main feature of soil microorganisms is the decomposition of their most complex high-molecular compounds into gases, water, and ordinary minerals. There are many microorganisms in the soil, that is, 1 g of soil contains millions or billions of microorganisms. Each soil type has its own microorganisms, with the number of microorganisms influencing the quality and quantity of organic matter, nutrients, and humus in the soil. This is much more than air and water. The soil contains various bacteria, actinomycetes, yeast, algae, and sodas, and scientists calculate that up to 3-5 tons of bacteria are found in the layer of arable land up to 25 cm deep. The number of microorganisms on the surface of the soil is high, and as they descend, their number decreases. Microorganisms are abundant in the 10-15 cm layer, as sunlight does not fall directly on it, nutrients and moisture are sufficient. In deep layers, these are rare because the soil acts as a natural filter and allows bacteria to penetrate groundwater less. Plant and animal remains are decomposed in the presence of microorganisms capable of absorbing cellulose, pentose, starch, pectin substances, and others, ultimately transforming into water with carbon dioxide [8].Ammonification is the process by which ammonia is formed as a result of the decomposition of proteins, amino acids, and other nitrogen-containing substances. This process is also called nitrogen mineralization. Because the resulting ammonia is transformed back into a mineral state using nitrifying bacteria. It has been established that proteins undergo ammonification in the presence of aerobic, anaerobic bacteria, and fungi. Among the microorganisms that exhibit particular activity in this process are representatives of the Pseudomonas family and representatives of the basil family [9]. Analysis of soil microorganisms revealed that the highest number of ammonifying bacteria was observed in section 5, according to which the number of ammonifying bacteria in 1 gram of soil in the 0-15 cm layer was 568*1010 CFU, in the 15-30 cm layer this indicator was 784*108 CFU, and in the 30-50 cm layer this indicator was 205*108 CFU. The minimum number of soil microorganisms in section 9 was 13*108 CFU in the 0-15 cm plow layer, 3*108 CFU in the 15-30 cm layer, and 48*107 CFU in the 30-50 cm subsoil layer per 1 g of soil. A total of 10 sections were obtained, according to which section 5 showed the highest values. The opposite was observed on the 9th segment. The reason for this can be seen in the amount of gypsum, salinity, and nutrients in the soils.Azotobacter (Latin. Azotobacter) is a genus of bacteria living in soil and capable of transforming gaseous nitrogen into a soluble form that can be absorbed by plants as a result of nitrogen fixation. The genus of nitrogen bacteria belongs to the class of gram-negative bacteria and belongs to the group of free-living nitrogen-fixing bacteria. Representatives of this genus live in neutral and alkaline soils, in water, and in cooperation with some plants [10,11].Analysis of microorganisms showed that the highest nitrogen-bacteric acid content in 1 g of soil was 648*1010 CFU in the 0-15 cm plow layer of section 5, 784*108 CFU in the 15-30 cm layer, and 205*108 CFU in the subsoil layer of 30-50 cm. The minimum nitrogen bacter content in the soil was 5*108 CFU in the 0-15 cm layer in section 4, 36*108 CFU in the 15-30 cm layer, and 43*107 CFU in the 30-50 cm layer. These analyses show that samples taken from section 5 yielded the best result, while in section 4, these indicators decreased slightly. Nitrobakteres increase the level of nitrogen in the soil by binding nitrogen, providing nutrients necessary for plant growth and development. Oligonitrophils are microorganisms that live in the soil and can survive in the presence of slightly bound nitrogen in the environment. Most of these organisms are diazotrops, meaning they can bind atmospheric nitrogen. These bacteria are anaerobic nitrogen-fixing microorganisms. Bacteria are capable of absorbing atmospheric nitrogen. Oligonitrophils are capable of participating in the process of atmospheric nitrogen fixation. They play an important role in the nitrogen cycle in nature, particularly in providing binding nitrogen to plants that are unable to absorb nitrogen from the air, but instead receive nitrogen after protein mineralization [12]. When studying the content of oligonitrophils from soil microorganisms in the sections taken for the study, the highest amount of 648*108 CFU was recorded in section 5 at 0-15 cm, while in the 15-30 cm section this amount was 158*108 CFU, and in the 30-50 cm layer it was 77*108 CFU. The lowest number of oligonitrophils in the plow layer of section 6 was 1*1010 CFU, 23*108 CFU in the 15-30 cm layer, and 7*108 CFU in the 30-50 cm layer. These results are due to their active participation in the assimilation of nitrogen-containing compounds and the integration of these substances into their metabolism.Nitrification is the process by which ammonia, formed by the decomposition of organic matter in soil, manure, and water, is oxidized to nitrite and then to nitrate. The first stage of nitration is carried out by representatives of five generations: Nitrosmonas, Nitrosococcus, Nitrosospira, Nitrosolobus, and Nitrosovibrio, while the second stage is carried out by representatives of the generations Nitrobakter, Nitrospira, and Nitrococcus. All these processes contribute to increasing soil fertility, improving soil composition, and increasing plant productivity. Analysis of microorganisms in 1 g of soil in the studied area showed that in section 6, the highest number of nitrificating bacteria in the plow layer at 0-15 cm was recorded at 1*1010 CFU, in the subsoil layer at 96*1010 CFU, and in the 30-50 cm layer at 33*108 CFU. The lowest nitrificator content was observed in section 5, where in the 0-15 cm layer it was 5*108 CFU, while in the next layer it was 2*1010 CFU, while in the 30-50 cm layer it was 8*107 CFU. Nitrificators provide nitrogen necessary for plants in the soil and increase soil fertility, while also helping to maintain a nitrogen balance in the environment. Nitrificators require the presence of ammonia or organic matter as a feed source. If the soil lacks these nutrients, the number of nitrificators may decrease.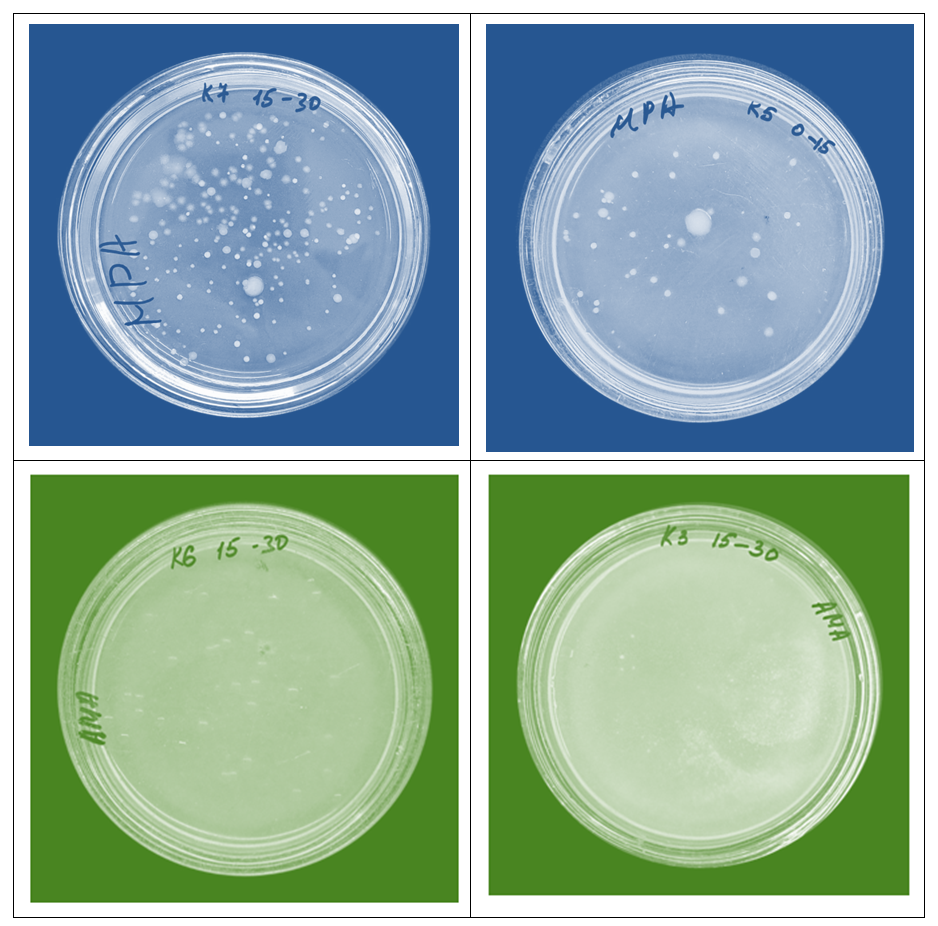 | Figure 2. Photographs of some microorganisms taken in the laboratory |
4. Conclusions
Among the studied groups of microorganisms, the predominance of ammonifiers was observed, followed by oligonitrophils, nitrogenobakteres, and nitrifying microorganisms. The change in the number of microorganisms along the depth of the layer can be explained by the lack of moisture in the soil and the low accumulation of organic matter along the profile. It has been established that the influence of gypsum content and salinity levels on the number of physiological groups of microorganisms in the studied soils varies. In terms of gypsum content, a decrease in the activity of microorganisms was observed in the direction of non-gypsum-low-medium-high soils.
References
| [1] | Gafurova L.A., Madrimov R.M., Razakov A.M., Nabiyeva G.M., Makhkamova D.Yu., Matkarimov T.R. Evolution, Transformation And Biological Activity Of Degraded Soils. International Journal of Advanced Science and Technology Vol. 28, no. 14, (2019), pp. 88-99. |
| [2] | Gafurova L.A, Makhamova D.Yu. Gypsum Soils of the Jizzakh Desert and Their Biological Activity “Innovative Development Publishing and Printing Hous” Monographiya T., 202.-184 p. |
| [3] | Minashina N. G. Melioration of saline soils. М.:Колос, 1978. -270 p. |
| [4] | Yamnova I. A. Pankova Y. I. Gypsum neoplasms and the elementary soil-forming processes forming them. Soil-science 2013г, № 12, - p. 1423–143. |
| [5] | Makhamova D.Yu., Iskhakova Sh.M, Abdullayeva X.B. The Effect of Easily Soluble Salts and Groundwater on gypsum formation in Gypsum Soils "Uzbekistan Soil" Scientific-Practical and Innovative Journal 2024. 2 / -26 p. |
| [6] | Mahkamova D. "Gypsum Soils of the Jizzakh Desert and Their Biological Activity" PhD Dissertation – Tashkent O’zMU. 2018. |
| [7] | Minashina N.G., Shishov L.L. Gypsum-bearing Soils: Distribution, Genesis, Classification. –J.:Soil-science 2002. 52 стр 52. |
| [8] | Makhkamova D., Gafurova L., Nabieva G., Makhammadiev S., Kasimov U., Julie M. Integral indicators of the ecological and biological state of soils in Jizzakh steppe, Uzbekistan. sustainable management of Earth resources and Biodiversity IOP Conf. Series: Earth and Environmental Science 1068 (2022) 012019 IOP Publishing doi:10.1088/1755-1315/1068/1/0120199. |
| [9] | Makhkamova D. Yu. Seasonal variation of ammonifier bacteria in heavy meliorated soils // International scientific and technical journal innovation technicaland technology. Vol. 2, No.1. 2021. ISSN: 2181-1067 Journal homepage: www.summusjournals.uz ISSN: 2181-1253. P. 54-58. |
| [10] | Gandora V., Gupta R. D., Bhardwaj K. K. R. Abundance of Azotobacter in great soil groups of North-West Himalayas // Journal of the Indian Society of Soil Science. - 1998. - Т. 46, № 3. - С. 379-383. |
| [11] | Martyniuk S., Martyniuk M. Occurrence of Azotobacter Spp. in Some Polish Soils // Polish Journal of Environmental Studies. - 2003. - Т. 12, № 3. - С. 371-374. |
| [12] | Zinchenko M.K. Stoyanova L.G Distribution of the Grouping of Oligotrophic Microorganisms in Agroecosystems of Gray Forest Soil // Vladimir Agricultural Journal - No 2(64) 2013. -p. 29-30. |




 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
