Norkobilova Z. B.
Karshi State University, Karshi, Uzbekistan
Correspondence to: Norkobilova Z. B., Karshi State University, Karshi, Uzbekistan.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This article presents the larvae of various stages, their abundance, and the dynamics of dragonfly larvae occurrence depending on the season in the water bodies of the Kashkadarya region. Additionally, the calculated data on the biomass and abundance of dragonfly larvae found in these water bodies are provided, and the significance of these important components in the aquatic biocenosis is discussed. In mid-April, with the onset of spring, Libellula quadrimaculata larvae were detected in the water bodies, followed later by Coenagrion pulchellum and Ischnura elegans larvae. By the end of April, it was found that the biomass of Libellula quadrimaculata larvae had reached its peak. The larvae of Libellula quadrimaculata become active in the first ten days of April, while Coenagrion larvae become active in the second ten days of April, leading to an increase in their biomass. By the end of April, the average biomass of Libellula quadrimaculata larvae was 2.7 kg, and the biomass of Coenagrion larvae was about 1.8 to 2 kg. From late April to early May, mature larvae of the genus Sympetrum began to appear. In May, it was noted that Ischnura larvae also started showing activity, and 3520 larvae of different stages were identified in a 300 m² water area at a depth of 50–70 cm. The biomass of Ischnura larvae amounted to 21,410 g. It was noted that the total mass of larvae of the main four generations in the basin was 1.5 kg. Analysis of the results shows that the larvae of the genus Sympetrum begin to show activity at a temperature of 11°C and become highly active at 23°C, with a density of 4.85 larvae per square meter.
Keywords:
Dragonflies, Odonata, Larval ecology, Reservoir
Cite this paper: Norkobilova Z. B., Biological and Ecological Characteristics Large, International Journal of Genetic Engineering, Vol. 12 No. 6, 2024, pp. 97-103. doi: 10.5923/j.ijge.20241206.06.
1. Introduction
Dragonflies are amphibious insects that predominantly inhabit aquatic environments during their larval stages [1,2,3,4,5,6]. Consequently, this research is centered on the ecological aspects of dragonfly larvae. Various scientific investigations have examined their ecology, morphology, and metamorphosis, including works by Popova (1953), Carvalho and Nessimian (1998), Steinhoff et al. (2016), Wonglersak et al. (2020), and Vilela et al. (2021). Popova’s (1953) study was particularly noteworthy in attempting to delineate the formation, structural dynamics, and population metrics of communities comprising dragonfly larvae species. [5,7]Geography and climate of Kashkadarya region. The Kashkadarya region spans 28.4 thousand square kilometers, representing approximately 7% of Uzbekistan's total area. Its geographical features are diverse, encompassing desert, semi-desert, hilly, and mountainous terrains. Research activities were concentrated in the developed zones of the Karshi Desert. These areas underwent substantial transformation in the 1970s due to human migration, which facilitated the establishment of agro-irrigation systems. The resultant artificial landscapes enhanced the ecological variability, leading to significant alterations in the natural environment. The expansion of irrigation systems supported the proliferation of mesophytic vegetation and contributed to the erosion-induced formation of ditch systems on saline lands. Regions such as Karshi, Kasbi, Nishon, and Mubarak emerged during the 1960s-70s, with the city of Karshi as a central hub. These ecological shifts created conducive habitats for dragonfly species, enabling their reproduction in desert settings. Particular attention was paid to the biological and ecological traits of dragonfly larvae during their life cycle studies in these areas. Fieldwork was conducted in the aquatic environments of Kasbi and Karshi districts. The region's aquatic systems, including ponds, reservoirs, and wetlands, experience rapid thermal dynamics due to intense solar radiation. Water temperatures, starting from 8°C in February, rise progressively to 18°C by April-May, aligning with the reproductive activity of spring dragonfly species. During the summer months (May-August), air and water temperatures peak at 30°C and 23°C, respectively. In September, temperatures decrease, with water temperatures ranging from 9–14°C, and continue to drop to near-zero by late October or November. As temperatures decline, larvae migrate to deeper, non-freezing zones for overwintering, with freezing conditions typically setting in between November 20 and December 1. The water bodies of Kashkadarya exhibit high biological productivity. Dragonfly larvae primarily consume zooplankton and benthos, with biomass measurements varying between 0.25–4.9 g/m² and 1.9–850.8 kg/ha. Benthos serves as a crucial food source for these amphibious insects, and reed-dominated still waters hold significant ecological importance. The described climatic and hydrological conditions favor the sustenance and development of both dragonfly larvae and adult populations. [1,2]
2. Materials and Methods
This study examined the species diversity, distribution, and density of dragonfly larvae across various aquatic environments in the Kashkadarya region. The research also assessed the abundance and biomass of larvae in selected model water bodies. Seasonal hydrobiological techniques were employed to capture the developmental dynamics of dragonfly species over extended periods. To estimate larval densities, the biocenometry method was utilized. Nine samples were collected from depths of 20–30 cm, 50 cm, and 70 cm in chosen water bodies. Sampling occurred every ten days between April and late June. In cases where water bodies dried up, additional samples were obtained from comparable sites, including flowing basins, swampy zones, and older water bodies situated at altitudes of 1,600–2,000 meters in mountainous areas. Samples were also collected along riverbanks, ensuring comprehensive spatial coverage. [4]
3. Results
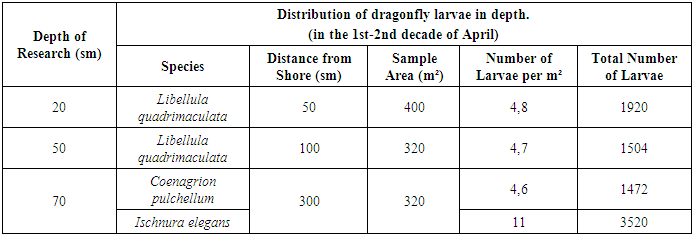
Larvae of Dragonflies in Reservoirs of Reclaimed Desert Areas: Distribution Across Depth Layers in the Kasbi and Karshi Districts Basin. This study investigates the seasonal distribution and habitat preferences of dragonfly larvae in reservoirs of reclaimed desert areas. By examining the larvae's spatial distribution and biomass during spring and early summer, the research sheds light on their ecological adaptations in arid environments. Field studies were conducted during the spring and early summer seasons. Observations began in early March, focusing on open water near the shore. Sampling was carried out using hydrobiological nets at various depths (20–70 cm) and distances from the shore (50–300 cm). Recorded parameters included larval density, biomass, and developmental stages. Samples were collected both from the shore and using a boat to access deeper areas.The first detection of Libellula quadrimaculata larvae occurred in the first ten days of April at a depth of 20–30 cm, approximately 3 meters from the shore. At this depth, the larval density was recorded at 4.8 individuals per square meter. Across an area of 400 m², a total of 1,920 larvae were observed. The average weight of each larva was determined to be 425 mg, resulting in a cumulative biomass of 816,000 mg. At greater depths, such as 50 cm and 70 cm, no larvae were found during the same period.The warmer near-shore water provided favorable conditions for dragonflies preparing for flight. By mid-April, the biomass of Libellula quadrimaculata larvae in the reservoir increased significantly, reaching approximately 6.0–6.5 kg.Continuing the phenological observations, habitats of dragonfly larvae in their later developmental stages were explored. Sampling was conducted using a hydrobiological net at a depth of 1–1.5 meters, approximately 10 meters from the shore. Larvae of Coenagrion pulchellum (family Coenagrionidae) were identified during this phase. The larvae of these dragonflies were found among the remnants of last year's Typha latifolia leaves in groups of 4–8 individuals. Sampling during the second and third ten-day periods of April revealed increased activity among Coenagrion larvae (see Table 1). [8]Table 1. Dragonfly Larvae Density Index in Water Bodies
 |
| |
|
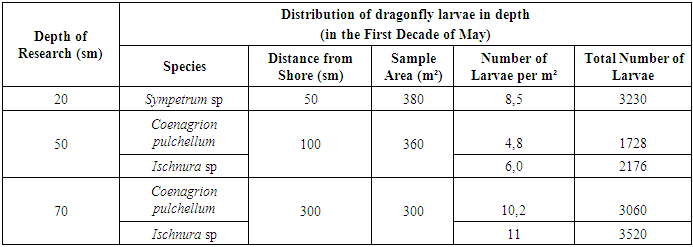
By the end of April, these larvae began migrating toward emerging hygrophytic vegetation, as the vegetative organs above the water were significantly warmed by solar radiation, providing an optimal habitat for the transition to their winged form. The biomass of Coenagrion larvae in the pond was recorded at approximately 1.8–2.0 kg.This study highlights the depth-specific distribution and biomass dynamics of dragonfly larvae in desert reservoirs, emphasizing the influence of seasonal and environmental factors on their developmental stages. [5]The study highlights the seasonal and spatial distribution of dragonfly larvae in reclaimed desert reservoirs. Larvae exhibited distinct depth and habitat preferences influenced by temperature, vegetation, and developmental stages. These findings provide insights into the ecological roles and developmental requirements of dragonflies in arid ecosystems.During late spring, most larvae of species belonging to the suborder Zygoptera were concentrated in the deeper sections of the water bodies, demonstrating their preference for cooler and more stable environments. Reduction and Emergence of Libellula quadrimaculata. By the end of April, the larvae of Libellula quadrimaculata had reached their final developmental stage. At this point, their average biomass decreased to 2.7 kg, marking a notable reduction from mid-April levels. The decrease in biomass coincided with the emergence of adult dragonflies (imagos), signaling the onset of the species' emergence period. The total biomass of dragonfly larvae in the basin by early May was calculated to be 4.3 kg. Developmental Progression of Sympetrum Larvae. Continuous monitoring throughout the spring revealed larvae from the Sympetrum genus at various developmental stages by late April and early May. Larvae in the 4th to 8th stages from the previous year's generation were identified. Additionally, larvae that had overwintered in the egg stage began to appear in May. Analysis showed that 11% of these overwintered larvae were at the 4th–6th stages of development, while the majority (89%) had progressed to the 7th–8th stages. The types and quantities of larvae identified in early May are detailed in Table 2. The observed reduction in Libellula quadrimaculata biomass indicates the maturation and emergence of adult dragonflies, a critical phase in the life cycle. The prevalence of overwintered Sympetrum larvae at advanced stages highlights the species' adaptation to the region's seasonal conditions. This stage-wise progression, coupled with habitat preferences, underscores the complex ecological strategies dragonflies employ to complete their life cycles. [3,5]Table 2. Dragonfly Larvae Density Index in Water Bodies
 |
| |
|
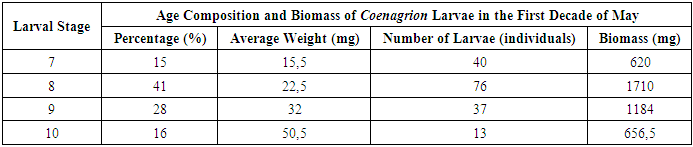
In May, larvae of the Ischnura genus began to exhibit increased activity. At depths of 50–70 cm, a population of 3520 larvae at various developmental stages was recorded within an area of 300 m². Distinct habitat preferences were observed among genera: - Sympetrum larvae were predominantly found in shallow waters (0–20 cm depth). - Coenagrion larvae were concentrated in deeper zones (50–70 cm depth).The density of larvae across the study sites ranged from 4.8 to 10.2 individuals per square meter, indicating significant spatial variability in larval distribution. Age Composition and Biomass of Coenagrion Larvae. An analysis of Coenagrion larvae revealed differences in their developmental stages and biomass at varying depths: - 56% of the larvae were at the 7th–8th stages of development. - 44% had progressed to the 9th–10th stages (Table 3). Table 3. Frequency of Occurrence of Coenagrion Larvae in Water Bodies
 |
| |
|
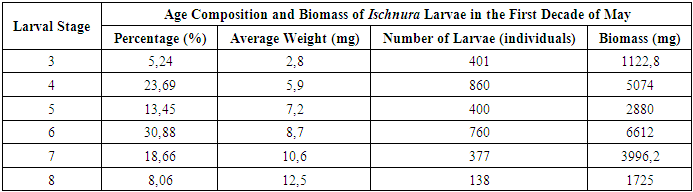
A positive correlation was observed between larval development stage and biomass: - Larvae at the 7th stage weighed an average of 15.5 mg. - Those at the 8th stage had an average biomass of 22.5 mg. - Larvae in the 9th–10th stages exhibited weights ranging from 32 mg to 50.5 mg. The majority of Coenagrion larvae in the study area were in the 8th stage of development, suggesting this stage represents a key period of growth within the population. The activity patterns and habitat preferences observed in Ischnura, Sympetrum and Coenagrion larvae highlight the ecological niche differentiation among these genera. The dominance of Coenagrion larvae at intermediate depths, combined with their advanced developmental stages, emphasizes the importance of these zones in supporting larval growth. The substantial increase in biomass across stages further underscores the energy demands associated with larval maturation, particularly during the later stages of development.During this period, we also conducted an analysis of the biomass of Ischnura larvae, examining their age composition and density at different water levels. This analysis was based on samples collected from the study areas, with the results presented in Table 4. The data provided insights into the growth patterns and distribution of Ischnura larvae across varying depths within the water bodies. [9] Table 4. Index of Occurrence of Ischnura Larvae in Water Bodies
 |
| |
|
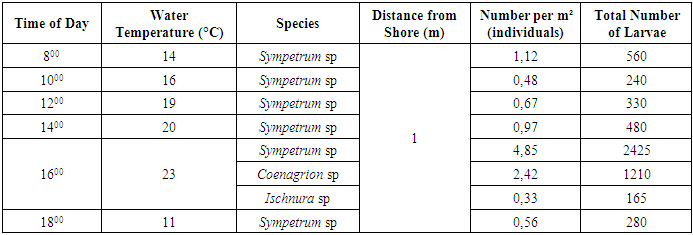
The analysis of Ischnura larvae revealed significant variations in both their age composition and biomass distribution across different stages. The results showed that larvae in the 3rd developmental stage represented the smallest proportion of the total biomass at 5.24%. In contrast, larvae in the 4th and 6th stages had the highest biomass percentages, ranging from 23.69% to 30.88%. When examining the number of larvae, it was found that there were 401 larvae at the 3rd stage and 400 larvae at the 5th stage, accounting for 13.45% of the total biomass. By May, the total biomass of Ischnura larvae in the study area had reached 21,410 g. Furthermore, the combined biomass of the four main generations of larvae in the basin was 1.5 kg. As the water temperature increased, we observed that dragonfly larvae began migrating towards the shallower areas near the shore, a phenomenon consistent with known behavioral patterns. Throughout the day, we collected samples from the shoreline for analysis, as summarized in Table 5. [2]Table 5. Daily Dynamics of Larvae Density on the Shores of Water Bodies (April 16, 2022)
 |
| |
|
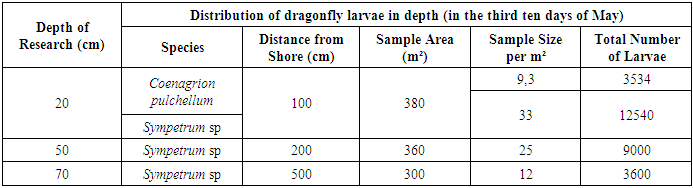
For this study, samples were collected from an area of 5 m² to analyze the temporal distribution and behavior of dragonfly larvae. The results indicated distinct activity patterns based on water temperature: - Sympetrum larvae were observed from 08:00 to 14:00, whereas Coenagrion and Ischnura larvae began appearing at 16:00, once the water temperature reached 23°C. These larvae were not found when the temperature decreased later in the day. - At 18:00, Sympetrum larvae were recorded at a density of 0.56 individuals per m², with a higher concentration around the water bodies, likely due to the decreasing temperature. The data shows that Sympetrum larvae exhibit a temperature-dependent activity pattern: they become active at 11°C and are highly active at 23°C, with a density of 4.85 individuals per m². Consequently, the highest migration of larvae to the shore occurred in the second half of the day when the water temperature reached 23°C (at 16:00), with the majority of migrating larvae belonging to the Sympetrum genus. [8] By the third decade of May, a uniform distribution of dragonfly larvae was observed across the study area, accompanied by a significant increase in their total biomass (see Table 6). Table 6. Dragonfly Larvae Encounter Rates in Water Bodies (Third Decade of May)
 |
| |
|
Sympetrum larvae were the most numerous and were found at all observed water depths. As development progressed, the larvae accumulated in the shallowest regions of the basin, very close to the shore. The distribution of Sympetrum larvae by age and biomass at the final stages of development is detailed in Table 7. During this period, the total biomass of Sympetrum larvae in the water bodies reached approximately 4.5 kg. In contrast, Coenagrion larvae, which were nearing the completion of their larval stage and undergoing metamorphosis, were less abundant in the water bodies. A total of 78,365 Coenagrion larvae were recorded, with a combined biomass of 2.5 kg (2,516,320 mg). The average weight of Coenagrion larvae was 32 mg. By the beginning of summer, it was observed that Sympetrum larvae remained present in the water bodies, continuing their development as they transitioned into the next stages of their life cycle.Table 7. Occurrence Rate of Sympetrum Larvae in Water Bodies
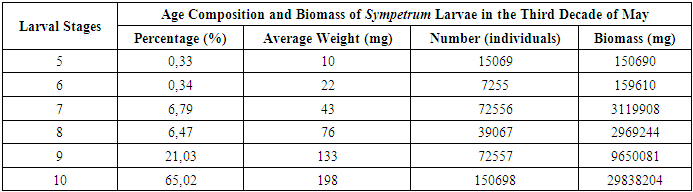 |
| |
|
As shown in Table 8, the larvae of this generation were evenly distributed across all depth layers of the basin, with their distribution influenced by their age composition. The total biomass of these larvae, across a 500 m² area (with an average density of 23 larvae per m² and a total of 11,500 larvae), was approximately 1.6 kg. This distribution highlights the adaptation of larvae to varying water depths as they progress through different developmental stages. [10,11]Table 8. Occurrence Rate of Sympetrum Larvae in Water Bodies
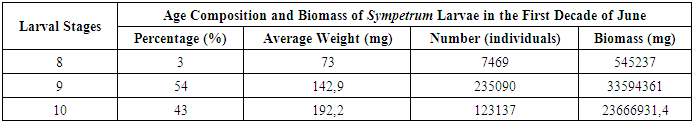 |
| |
|
Migration and Emergence of Sympetrum larvae. By this time, the migration of Sympetrum larvae towards the shore had been observed as they transitioned into the adult stage. These larvae moved toward the stems and leaves of plants, such as reeds, which grow 1.5–2 meters above the water surface. These plants provided a substrate for their next developmental stage. The substrates were primarily located in the coastal areas of the basin. Remnants of larval shells could be found on the vegetation along the basin's edges. From mid-June, Sympetrum larvae became abundant along the shores, marking the beginning of their emergence period. At this point, the larvae's biomass was relatively small, totaling 182 g. By the end of June, a decline in the number of dragonfly larvae in the basin was observed. During this period, it was noted that Coenagrion larvae had completed metamorphosis, and adult insects had emerged. The Ischnura and Sympetrum genera, however, had a relatively prolonged adult emergence period compared to other genera. In July, the emergence of the second generation of adults from this cohort was observed. [3,6]The Larvae of Dragonflies on the Mountainous Riverbanks. The data from the water bodies in the studied areas provide a general overview of the characteristics of dragonfly larvae during their developmental stages. However, this data remains incomplete without considering the water bodies in the mountainous regions of the Kashkadarya area. In 2021, we conducted practical research around the Kizil River in the Yakkabog district of the Kashkadarya region. The research took place in August, which is the middle of summer in this arid region, according to the phenological patterns of the mountainous areas. The study area is situated at an altitude of 1500 meters above sea level, in a valley between mountains. The temperature during the summer reaches +30°C, while in winter, the lowest temperature can drop to -10°C. The warm season lasts for about 100 days, and the annual precipitation averages 600 mm, with snow cover ranging from 15-20 cm to 100 cm. We selected the banks of the Kizil River as the study site. The riverbed contains marshy areas with aquatic plants, including reeds, rushes, cattails, and horsetails. Further from the riverbank, dragonfly larvae were not found due to a lack of nutrients and the increasing water flow. A biocenometry study was conducted on a 500 m² area of the riverbank, predominantly covered with reed thickets, and the following findings were made:- Sympetrum species of dragonflies were recorded as the dominant species, with a population density of 15 larvae per square meter, totaling 750 larvae in the reed plants.- Coenagrion species larvae were also present in the area.- The banks of the river are covered with various aquatic plants, providing a suitable habitat for Sympetrum larvae. The water depth in this area ranged from 10 to 50 cm. Overall, it was estimated that there were about 76,800 larvae living in the area, with a density of 64 larvae per square meter.The warmth of the water and the abundance of aquatic plants create favorable conditions for dragonfly larvae development. However, the limited surface area and the competition for resources, particularly among closely related species that dominate ecologically, add complexity to their survival. Our observations showed that Coenagrion larvae, preparing for overwintering, were located in the deeper parts of the water body, at a depth of 4–6 meters. This finding provides valuable insights into the environmental conditions affecting dragonfly larvae in mountainous regions, particularly along the Kizil River in Kashkadarya. The selection of August for the study aligns with the seasonal changes in the region, which influence the aquatic habitats where dragonfly larvae thrive. The data highlights the dominance of Sympetrum larvae in specific microhabitats, particularly in reed-covered marshy areas. This species has adapted well to the available environmental conditions, given its high larval density. Meanwhile, the presence of Coenagrion larvae in deeper areas of the river suggests the existence of different ecological niches, potentially shaped by factors like water temperature, depth, and plant cover. Moreover, the study sheds light on the competitive dynamics within these aquatic environments. As resources become limited in smaller areas, competition intensifies, particularly between species occupying similar ecological roles. This competition may significantly affect the population structure and survival strategies of dragonflies in these habitats. The study also points to adaptation mechanisms used by species like Coenagrion, which appear to migrate to deeper waters as a strategy for preparing for overwintering. The findings contribute to a broader understanding of how dragonfly larvae, particularly Sympetrum and Coenagrion species, adapt to the ecological conditions of mountainous riverbanks. Further research could explore the influence of changing environmental factors, such as water flow and temperature, on the behavior and survival of these species. [10]
4. Discussion
The findings from this study provide valuable insights into the behavior, abundance, and migration patterns of dragonfly larvae in aquatic ecosystems. The study revealed that the dynamics of dragonfly larvae abundance were closely linked to seasonal changes, water temperature, and depth, with significant shifts observed during the spring and summer months.Seasonal Abundance Patterns. The highest abundance of dragonfly larvae, particularly those from the Sympetrum genus, occurred during the second decade of May and the first decade of June. This period corresponds to the pre-emergence phase, where larvae are in their final developmental stages before metamorphosis. During this time, larvae were found to constitute up to 53% of the total biomass of aquatic organisms in the study area, indicating their ecological significance in these ecosystems. This high abundance is characteristic of the peak period of larval development across all water depths. As the larvae matured and began to emerge, their biomass and relative abundance within the aquatic community declined, suggesting that the dragonfly population shifts from a dominant presence to a less significant role as they transition into the adult stage. [2,9]Depth-Dependent Distribution. The study highlighted that dragonfly larvae were distributed at varying depths within the water body, with differences observed in the Sympetrum, Coenagrion and Ischnura species. The presence of larvae at both shallow and deep water layers suggests that different species have specific preferences for their developmental environment. Sympetrum larvae were predominantly found near the shore, whereas other species like Coenagrion were located at deeper areas. This depth-dependent distribution is likely a strategy for maximizing survival and minimizing competition. Shallow areas, which offer rich vegetation and warmer temperatures, provide an ideal environment for Sympetrum larvae, while deeper areas provide protection and stability for species like Coenagrion during their development. [7,10]Migration Dynamics. The study's analysis of the daily migration patterns of larvae to the shore offers significant insights into the behavioral ecology of dragonfly larvae. The migration of dragonfly larvae from deeper water to the shore was strongly influenced by water temperature. The majority of larvae migrated during the late afternoon, with a peak migration time between 4:00–6:00 PM. This peak coincided with the warmer temperatures of the day, suggesting that larvae may be seeking optimal conditions for their developmental and physiological needs. The fact that Sympetrum larvae made up the majority (75.83%) of those migrating further supports the dominance of this genus in the study area, particularly in the shallow regions near the shore. This migration is likely a precursor to the larvae’s emergence into adult dragonflies. The shore areas, particularly those with aquatic plant cover like reeds and cattails, provide the necessary substrate for the larvae to undergo their final metamorphosis. The migration behavior also indicates an adaptive response to environmental changes, such as water temperature, which can influence the timing of development and the success of metamorphosis. [1,8,11]Ecological Implications. The findings of this study have important ecological implications. The dominance of Sympetrum larvae, particularly in shallow areas near the shore, suggests that this species is well-adapted to the specific conditions of the study area. These areas provide favorable conditions for growth, such as warmer temperatures and abundant vegetation. The migration of larvae to the shore, especially in response to rising temperatures, may also reflect the importance of temperature regulation for successful metamorphosis. Additionally, the migration behavior observed during the study period may be indicative of the larvae's search for the optimal sites for their next developmental stage. This movement pattern is essential for understanding the environmental cues that drive dragonfly life cycles and can inform conservation efforts, particularly in areas where habitat degradation or climate change may impact water temperatures and available substrate for larval development.Competition and Habitat Utilization. The study also highlighted the competitive dynamics among dragonfly species, particularly in areas with limited space and resources. As the density of larvae increases, competition for space, food, and optimal developmental sites intensifies, especially among closely related species like Sympetrum and Coenagrion. This competitive pressure likely influences the distribution and success of each species, with some larvae migrating to deeper areas for overwintering, as observed with the Coenagrion genus. Understanding these competitive interactions is crucial for predicting how species will respond to changes in their environment, such as altered water flow or temperature fluctuations. [11]
5. Conclusions
In conclusion, this study offers a comprehensive look at the dynamics of dragonfly larvae, particularly in relation to seasonal abundance, migration behavior, and habitat utilization. The findings underscore the importance of water temperature, depth, and aquatic plant cover in shaping the life cycle and distribution of dragonfly larvae. Future research could expand on these findings by exploring how environmental factors such as water quality, climate change, and habitat alterations affect dragonfly populations and their migration patterns. Furthermore, studies on the interaction between dragonfly species and other aquatic organisms will contribute to a better understanding of the ecological role of dragonflies in freshwater ecosystems.
References
| [1] | Chernychev, V.B. Ecology of Insects. Moscow: Moscow State University, 1996. pp. 15-304. |
| [2] | Yakhontov, V.V. Ecology of Insects. Moscow: Higher School, 1969. 488 pp. |
| [3] | Machado, A.B.M. Peruviogomphus belle spec. nov. from the Amazonian region of Brazil (Anisoptera: Gomphidae). Odonatologica, 34 (1), 2005. pp. 59-63. |
| [4] | Yang, G.H., Orr, A.G., & Zhang, H.M. First description of the larva of Archineura incarnata (Karsch, 1891) with notes on the biology (Odonata: Calopterygidae). Zootaxa, 5134 (3), 2022. pp. 441-447. DOI: 10.11646/zootaxa.5134.3.8. |
| [5] | Vilela, D.S., Venâncio, H., & Santos, J.C. Morphological description of the final instar larvae of Argia reclusa Selys, 1865 and Tigriagrion aurantinigrum Calvert, 1909 from Southeastern Brazil (Odonata: Coenagrionidae). Zootaxa, 5060 (3), 2021. pp. 392-400. |
| [6] | Popova, A.N. Dragonfly Larvae of the USSR Fauna (Odonata). Leningrad: Academy of Sciences of the USSR, 1953. 235 pp. |
| [7] | Carvalho, A. & Nessimian, J. Odonata of the State of Rio de Janeiro, Brazil: habitats and habits of larvae. Ecology of Aquatic Insects. Series Oecologia Brasilensis, Rio de Janeiro, Brazil: PPGE-UFRG, 1998. pp. 3-28. |
| [8] | Steinhoff, P.O., Butler, S.G., & Dow, R.A. Description of the final instar larva of Orthetrum borneense Kimmins, 1936 (Odonata, Libellulidae), using rearing and molecular methods. Zootaxa, 4083 (1), 2016. pp. 99. |
| [9] | Yang, G.H., Orr, A.G., & Zhang, H.M. First description of the larva of Archineura incarnata (Karsch, 1891) with notes on the biology (Odonata: Calopterygidae). Zootaxa, 5134 (3), 2022. pp. 441-447. DOI: 10.11646/zootaxa.5134.3.8. |
| [10] | Popova, A.N. Dragonfly Larvae of the USSR Fauna (Odonata). Leningrad: Academy of Sciences of the USSR, 1953. 235 pp. |
| [11] | Wonglersak, R., Fenberg, P.B., Langdon, P.G., Brooks, S.J., Price, B.W. Temperature-body size responses in insects: a case study of British Odonata // Ecological Entomology. 45(4), 2020. -P. -795-805. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML