Sultonova K.1, Kushiev H. H.2
1Samarkand State University of Veterinary Medicine, Animal Husbandry and Biotechnology, Uzbekistan
2Gulistan State University, Uzbekistan
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
In recent years, possibilities have been achieved based on the widespread use of in vitro methods in the preservation and development of biodiversity. The specificity of the morphogen response to the effects of growth regulators of the studied samples of Lagochilus inebriance in in vitro condition was determined using mineral nutrient media with different composition. The optimal medium was selected for the micropropagation of the studied L.inebriance samples; The addition of 0.1 μM BAP to the nutrient medium according to recipe B5 was noted to be effective; BDS medium containing 5.0 µM BAP and 2.0 µM NAA was used for samples of L. inebriance growing in laboratory conditions, and 0.4 µM BAP, 3.2 µM NAA for samples growing in greenhouse conditions and B5 nutrient medium supplemented with 2.3 μM IAA was noted to be the most effective. In the proliferative phase, the most effective cytokinin was BAP compared to TDZ and Kinetin.
Keywords:
Lagochilus inebriance, In vitro, Microclonal propagation, Callus, MS medium, Phytohormone
Cite this paper: Sultonova K., Kushiev H. H., Obtaining Pathogen-Free Seedlings Based on Microclonal Propagation of Lagochilus Inebriance Bunge Using in Vitro Methods, International Journal of Genetic Engineering, Vol. 10 No. 1, 2022, pp. 10-15. doi: 10.5923/j.ijge.20221001.03.
1. Introduction
In recent decades, environmental changes and the worsening of the situation, negative changes in the natural environment and the increase of anthropogenic influence, as well as the reduction of resources due to the unplanned use of natural resources, and this situation also caused the decrease in the diversity of species and their number. With the loss of a single plant species, we lose not only a component of the world's flora, but also a potentially valuable genetic resource, causing changes in plants. Because each species is a producer of biologically active compounds related to the growth and development of another species [1,2]. This is an important life process that expresses the interdependence of existing genetic diversity.Accordingly, two strategic systems are used in connection with the preservation of genetic diversity. These are in situ - in natural ecosystems created in specially protected natural areas (SPNA): nature reserves, reserves, national parks, natural monuments, etc. and ex situ - outside natural habitats: collections of botanical gardens, gene banks [3].It should be noted that in situ conservation of biodiversity is the most important, but this method is not always suitable for the conservation of certain species. Therefore, ex situ strategies for gene pool conservation are becoming increasingly popular. Development of genetic biodiversity in situ is important in increasing ex situ opportunities [4,5].In solving the above problems, it is possible to use biotechnology methods, that is, to grow isolated cells, tissues and organs of plants in an artificial nutrient medium and to create a new gene bank in vitro.The biotechnological approach has several advantages over traditional methods of conservation of endangered species [6]. In the same way, this approach allows to restore the number of protected taxa by creating artificial populations on the territory of natural zones [7]. One of the most relevant advantages of in vitro conservation is the possibility of obtaining sterile cultures of species without removing them from their natural habitats, which prevents the destruction of phytocenoses [8]. This is one of the urgent issues, especially in the works on the preservation of genetic resources of plants based on in vitro methods for the collection of natural populations and further cultivation and collection as medicinal raw materials [9].In this work, the results of the research on obtaining pathogen-free seedlings in vitro and creating a plant plantation in order to preserve the Lagochilus inebrians plant belonging to the genus Lagochilus, which has medicinal properties, are described.
2. Research Object and Methods
Lagochilus inebrians, chosen as a research object, belongs to the genus Lagochilus and belongs to the family of labial flowers (Lamiaceae or Labiamae). There are 44 species of this family in the world. Of these, 25 species are found in Central Asia, and 17 species are found in Uzbekistan [10]. The demand for Lagochilus preparations is increasing year by year, while the natural reserves of wild Lagochilus are rapidly decreasing.L.inebrians grows as a weed in foothills, low mountains, gravel and river tributaries, and sometimes on the banks of canals and ditches. Mainly distributed in Samarkand, Jizzakh, Navoi regions of Uzbekistan and some other republics of Central Asia [10].In connection with the widespread use of the Lagochilus inebrians plant as a medicinal plant and the depletion of its natural resources, we have conducted research on in vitro micropropagation using biotechnological methods. Nutrient media and growth regulators for in vitro cultivation of L. inebriance explants were selected.1. At the first stage of in vitro cultivation, experiments were carried out in 4 variations with two different ratios of growth regulators to MS [11] and VM [12] nutrient media (table 1).2. Explants of 50 per variant were performed in triplicate. Explant viability (%) and their morphogenetic activity (%) were taken into account.Table 1. Concentrations of nutrient media and growth regulators for the cultivation of L.inebriance explants
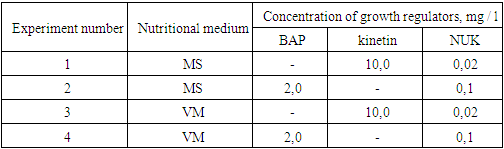 |
| |
|
To determine the effect of decreasing and increasing concentrations of growth regulators on the viability of explants, they were grown in MS medium with BAP range from 2 to 6 mg/L and NSC from 0.01 to 0.2 mg/L. The experiment was carried out in three repetitions with a total of 10 variants. As a control, nutrient medium without growth regulators was taken. The experiment was carried out considering the viability (%) and morphogenetic activity (%) of the explants.BAP and NSC were systematically studied based on a reduced concentration range and closer concentration values. In this case, 8 options were studied in three variants in the MS environment (table 2).Table 2. Concentration of growth regulators to activate morphogenesis in L.inebriance explants
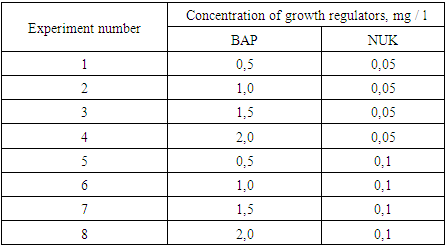 |
| |
|
Viability (%), morphogenetic potential (morphogenic explants of the total number of cultured explants) and shoot proliferation capacity (number of shoots/shoots per explant) were evaluated in the analysis of BAP and NSC effects.Effect of kinetin during the introduction of explants into in vitro culture. MS nutrient medium containing BAP, NSC and kinetin at different concentrations was used. In 6 variants of the experiment, 3 repetitions were determined (3 tables).Table 3. Concentrations of growth regulators for morphogenesis induction in L.inebriance explants
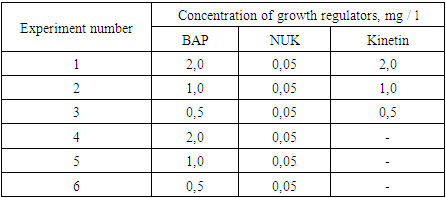 |
| |
|
The morphogenesis ability of the explants was analyzed by determining the percentage of morphogenic explants from the total number (%), length (mm) and number of leaves (pieces) of the cultured explants.Statistical processing of experimental data was carried out using the methodological recommendations specified in the book of B.A. Dospekhov [13], as well as the methods presented in the workbook "Basics of Scientific Research in Agronomy" (1985). The most voluminous calculations were made using the Microsoft Office Excel program.
3. Results and Analysis
Studies on in vitro tissue culture as primary explants were conducted on L. inebriance grown under greenhouse isolation conditions. The high level of contamination of underground organs led to the need to use strict sterilization regimes. Using a solution of 70% ethanol (30 sec), 0.1% mercury(II) chloride, and Tween 80 (30 min) in the workup, followed by washing with sterile water, was found to provide a higher percentage. The tissue sterilization method used was effective, yielding uninfected explants ranging from 87-96%.At the initial stage of in vitro culture, agar nutrient medium supplemented with 5.0 μM BAP with 2.0 μM NAA according to BDS and B5 recipe was used. It was found that the use of nutrient mediums containing plant growth regulators stimulated regeneration processes in the tissues of L. inebriance primary explants compared to medium without control hormones [14]. In media supplemented with growth regulators, the frequency of random microbud renewal ranged from 49.0 to 76.9%, and the average number of shoots formed de novo was 2.0 to 3.6/exp. At this stage, L.inebriance segments were found to have less regenerative potential among other studied species (Table 4).Table 4. Effects of nutrient medium components on adventitious shoot regeneration of L.inebriance
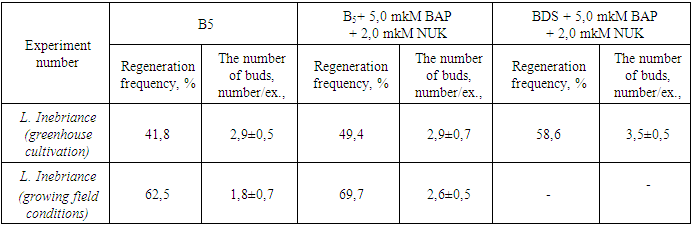 |
| |
|
The species difference in the rate of morphogenic reaction was determined. The first changes on the surface of shoots, that is, a slight growth of explant tissue, were noted after 15-17 days of L. inebriance grown in isolated conditions in the greenhouse, and in 51-56 days under field conditions. The regeneration of adventitious shoots, from the appearance of a tumor on the explant surface one day after inoculation to the formation of primary vegetative organs, took an average of 12-17 days.At the initial stage of in vitro cultures, the development of callus tissue did not occur, and shoot regeneration continued directly with the path of organogenesis. The initiation of buds was noted in the intact part of the tissue above the surface of the nutrient medium, but their formation occurred closer to the surface of the injured (cut) area (Fig. 1). At the same time, accidental division of buds into segments, i.e. tissue damage, caused regeneration processes to occur. In natural conditions, the formation of random buds also occurs as a result of injured/cut tissue or secondary lateral meristem activity [15]. However, more active regeneration was not recorded in the donut area. Accordingly, our results differ from those obtained by other researchers.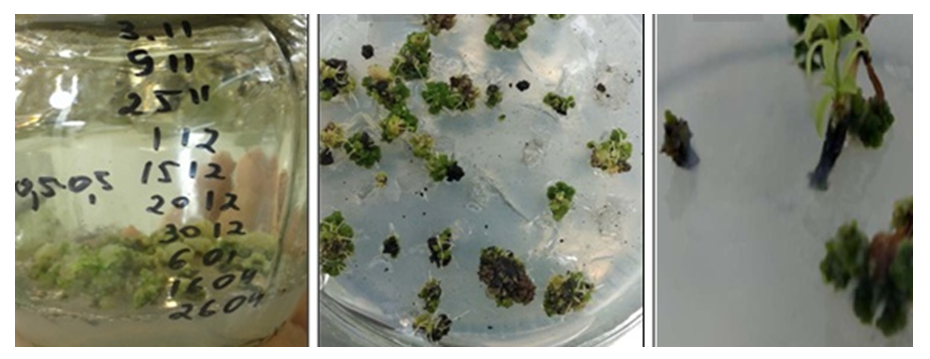 | Figure 1. Regeneration of L.inebriance shoots on primary explant in BDS medium supplemented with 5.0 μM BAP and 2.0 μM NAA, 47 days after inoculation |
Selection of the optimal nutrient medium according to the combination of mineral composition, concentration and growth regulators is the main factor that promotes the development of existing meristems or their new formation in the plant [16]. During the proper reproduction phase, regeneration of L. inebriance microbuds proceeded synchronously: by the end of the transition, they had similar sizes in one conglomerate (Fig. 2). In 35-40 days, microbuds reached 3.0-4.5 mm in diameter. In order to provide next growth, the duration was increased to 50-55 days or they were transferred to a new hormone-free medium. | Figure 2. L. inebriance conglomerate shoots on BDS medium without growth regulators, 29 day after inoculation |
Based on the analysis of variance data, it was not possible to determine the most important factors affecting the number of shoots formed during the actual reproductive phase (Table 5). However, BDS and B5 media were characterized by similar regeneration rates of L. inebriance microshoots. A decrease was noted in the MS medium. Taking this into account, further work was carried out using BDS and B5 mineral bases (Table 5). Similar results were obtained in the cultivation of other studied species.In BDS medium without control hormone (regeneration - 42.7%, the number of shoots - 4.1±0.2 units/ex.) high rates of growth and development of shoots were observed. This can be explained by the accumulation of growth regulators in buds applied at the initial stage of in vitro culture [14]. In general, the introduction of exogenous growth regulators into the nutrient medium during the reproductive phase was accompanied by a slight increase in the activity of L. inebriance bud formation in vitro. However, in some versions, the regeneration activity was lower than in the control medium.It was found that the addition of growth regulators 5.0 μM BAP and 2.0 μM NAA to BDS medium resulted in the formation of additional shoots on day 12-15 of culture. In BDS nutrient medium without hormones, this period was 22-25 days. In this case, the average number of shoots per explant is 4.6±0.4, the frequency of regeneration is 56.3%. Higher rates of growth and development were also observed in BDS medium supplemented with 10.0 μM BAP and 2.0 μM NAA: the regeneration rate reached 66.2%, while random buds were formed per explant 4.0±0,8.Use of low concentrations (0.1 and 0.5 μM) of BAP, Kinetin, and TDZ resulted in the formation of yellow, morphogenic callus regardless of the mineral content of the medium. At the same time, the frequency of formation of morphogenic callus did not exceed 38.0%. The formation of buds on the callus surface was observed only after 5 weeks of cultivation. This is much later than in a medium filled with cytokinins and auxins. Later, adventitious buds emerged from these buds, i.e. indirect organogenesis continued. At the same time, only 28.0% of random buds became microbuds (Fig. 3). This was especially evident in BDS medium supplemented with 0.5 μM TDZ: despite the high percentage of regeneration and the number of laid shoots (up to 11), only 3.1±1.2 well-formed shoots resulted from the development of the conglomerate.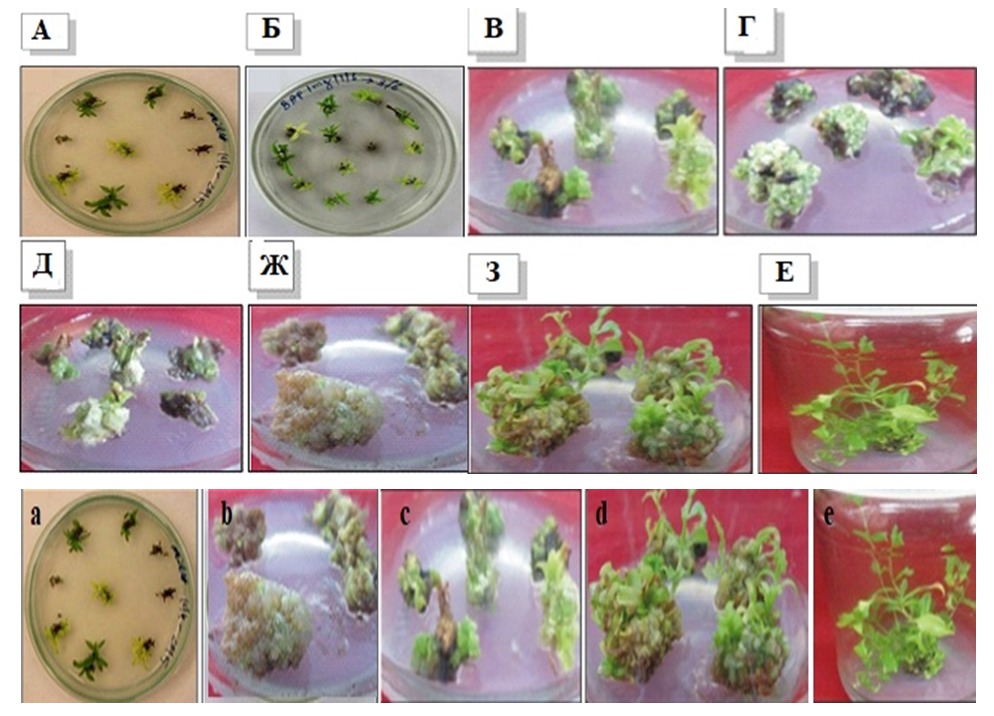 | Figure 3. Indirect regeneration of L. inebriance on the surface of morphogenic callus: a - callus formed from the tip meristimatic tissue of the stem, b - callus formed from the explant, c- development of the stem; d – stem regeneration process; e-microshoots ready for transfer to soil |
Although analysis of variance did not reveal a significant effect of environmental factors on the number of microbuds formed, the addition of 5.0 μM BAP and 2.0 μM NAA to BDS nutrient medium compared to control and medium supplemented with cytokinins alone we found that it accelerates the regeneration almost twice.The study revealed the effect of the mineral composition of the nutrient medium (B5 and BDS) on the regeneration activity of L.inebriance shoots and, at the same time, the significant effect of growth regulators on the number of adventitious shoots formed. Lower concentrations (0.1 and 0.5 μM) of BAP and TDZ were more effective. Addition of 0.1 μM BAP to the medium according to the recipe B5 resulted in the growth of explant tissues and the highest number of explants on its surface with a frequency of 56.5% (5.0 ± 1.5 units/ led to the formation of exp.). This allowed us to consider this medium as optimal (Fig. 4).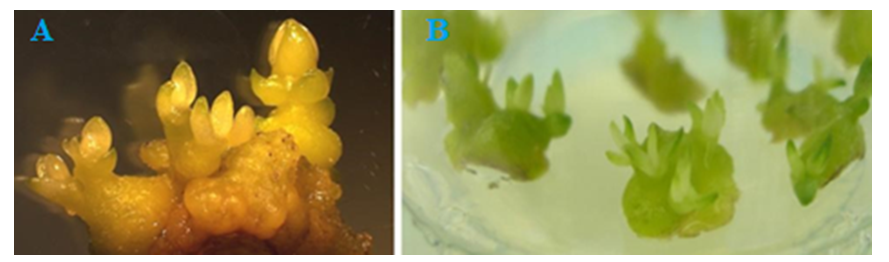 | Figure 4. Random buds formed in L. inebriance callus tissues: a – shoot regeneration on the surface of morphogenic callus, BDS medium supplemented with 0.5 μM BAP, 31 days after inoculation; b - Shoots formed on explant tissues grown, B5 medium supplemented with 0.1 μM BAP, day 23 |
The formation of yellow-green morphogenic callus (37%) was noted during cultivation in this nutrient medium. Its long development (more than 40 days) led to the formation of random microbuds. However, the frequency of shoot regeneration on the surface of morphogenic callus did not exceed 32%. Therefore, both direct and indirect regeneration were noted in this medium.Intensity of stages (callus tissue formation and proliferation; tumor formation, root formation), type of explant, sterilization of explant, nutrient medium depends on factors such as combinations of phytohormones. Combination of BAP (5 mg/l)+NAA (0.4 mg/l) in MS nutrient medium in the variant using leaf axil buds and leaf pieces as initial explants in the experiments was found as an optimal indicators for formation of callus tissue. Also, it was found that the intensity of callus tissue formation is high in the combination of BAP (3-4 mg/l)+NAA (0.4 mg/l) in MS nutrient medium when using shoots and leaf pieces located at the tip of the stem. In the experiments, it was found that the proliferation of callus tissue is carried out optimally in the combination of BAP (1 mg/l)+NAA (1 mg/l) in MS nutrient medium. It was also noted that the intensity of root formation was relatively high in the combination of BAP (2 mg/l)+NAA (0.1 mg/l)+GA3 (0.5 mg/l).MS and WPM nutrient medium were analyzed on a comparative basis during in vitro micropropagation of Lagochilus Inebrians. In this case, in the presence of a combination of kinetin (2.3-18.4 μM) + 1-naphthalene acetic acid (0.54 μM), it was noted that the regeneration intensity was higher in the WPM nutrient medium than in the MS nutrient medium.In the experiments, it was found that GK (0.5-2.0 mg/l) has a significant positive effect on the intensity of the process of rhizogenesis - organogenesis during culture. In addition, it was determined that the intensity of regeneration depends on the genotype of the plant, the type of nutrient medium, the stimulants and combinations used in its composition. In particular, when 6-BAP (1-2 mg/l)+GK (0.5-1 mg/l)+NSK (0.2 mg/l) is used in the composition of the Kvorin-Lepore nutrient medium, the regeneration process in the explant is sufficiently optimal.Seedlings obtained in vitro were gradually transferred to soil. To do this, the plant obtained from in vitro was planted on a substrate in a greenhouse. The substrate consists of two layers of sand and wood shavings. The top of the substrate is a simple substrate, the surface layer is covered with wood shavings. | Figure 5. Transfer of Lagochilus inebrians from in vitro conditions to soil |
It was found that Lagochilus Inebrians callus tissue proliferation under in vitro conditions is optimally realized in the combination of BAP (1 mg/l)+NAA (1 mg/l) in MS nutrient medium. In the combination of BAP (2 mg/l)+NAA (0.1 mg/l)+GA3 (0.5 mg/l), relatively high intensity of root formation was noted. In the process of microcloning, in the presence of a combination of kinetin (2.3-18.4 μM) + 1-naphthalene acetic acid (0.54 μM), it was noted that the regeneration intensity was higher in the WPM nutrient medium than in the MS nutrient medium. Methods of propagation of plant tissue using in vitro method were created for the Lagochilus Inebrians.
4. Conclusions
The specificity of the morphogenic reaction of the studied samples of L.inebriance to the effects of growth regulators was determined when using mineral medium of different composition. The optimal medium was selected for the actual reproduction of the studied L. inebriance specimens; the addition of 0.1 μM BAP to the nutrient medium according to recipe B5 was noted to be effective; a medium containing BDS was used for L.inebriance specimens grown under laboratory conditions. BDS medium containing 5.0 µM BAP and 2.0 µM NAA was used for samples of L. inebriance growing in laboratory conditions, and 0.4 µM BAP, 3.2 µM NAA for samples growing in greenhouse conditions and B5 nutrient medium supplemented with 2.3 μM IAA was noted to be the most effective. In the proliferative phase, the most effective cytokinin was BAP compared to TDZ and Kinetin.
References
| [1] | Rao, N.K. Plant genetic resources: Advancing conservation and use through biotechnology / N.K. Rao //Afr J Biotech. – 2004. – Vol. 3 (2). – P. 136-145. |
| [2] | Leung, D.W.M. Plant biotechnology helps quest for sustainability: With emphasis on climate change and endangered plants / D.W.M. Leung // Climate change and sustainable development (Ed. R. Reck). – Louisville: Linton Atlantic Books, 2010. – P. 247-250. |
| [3] | Maxted, N. Complementary conservation strategies / N. Maxted, B.V. FordLloyd, J.G. Hawkes // Plant genetic conservation. The in situ approach. (Eds. N. Maxted, B.V. Ford-Lloyd, J.G. Hawkes). – London: Chapman, 1997. – P. 15-41. |
| [4] | Benford G. An ex situ «Library of Life» strategy // Protection of global biodiversity converting strategies / Eds L.D. Guruswamy, J.A. McNeely. Durham, London Duke Univ. Press. 1998. P. 87–97. |
| [5] | Schuiteman A., de Vogel E.F. Taxonomy for conservation // Orchid conservation / Eds K.W. Dixon, S.P. Kell, R.L. Barret, P.J. Cribb. Kota Kinabalu, Sabah: Natural Publ., 2003. P. 55–68. |
| [6] | Reed, B.M. Biodiversity conservation and conservation biotechnology tools / B.M. Reed, V. Sarasan, M. Kane, E. Bunn, V.C. Pence // In Vitro Cell Dev Biol – Plant. – 2011. – Vol. 47. – P. 1–4. |
| [7] | Tandon, P. Prospects of plant conservation biotechnology in India with special reference to northeastern region / P. Tandon, S. Kumaria // Biodiversity: Status and Prospects (Eds. P. Tandon, M. Sharma, R. Swarup). – New Delhi, India: Norasa Publishing House, 2005. – P. 79-91. |
| [8] | Leung, D.W.M. Plant biotechnology helps quest for sustainability: With emphasis on climate change and endangered plants / D.W.M. Leung // Climate change and sustainable development (Ed. R. Reck). – Louisville: Linton Atlantic Books, 2010. – P. 247-250. |
| [9] | Slabbert, M.M. In vitro production of Lachenalia / M.M. Slabbert, J.G. Niederwieser // Plant Cell Rep. – 1999. – Vol. 18. – P. 620-624. |
| [10] | Т.И. Цукерваник. Система рода Lagochilus (Lamiaceae). Ботанический журнал. 1985г. Т. 70. с. 1183-1190. |
| [11] | Murasige T., Skoog F., 1962- Murashige, T. A revised medium for rapid growth and bioassays with tobacco tissue cultures / T. Murashige, F. Skoog // Physiol Plant. – 1962. – Vol. 15. – P. 473497. |
| [12] | De Bruyn, M.H. In vitro propagation of Amaryllis belladonna / M.H. De Bruyn, D.I. Ferreira, M.M. Slabbert, J. Pretorius // Plant Cell Tiss Organ Cult. – 1992. – Vol.31. – Р. 179–184. |
| [13] | Доспехов Б.А. (Методика полевого опыта (с основами стаптистической обработки результатов исследований). М. 1985. |
| [14] | Nas M.N., Gokbunar L., Sevgin N., Aydemir M., Dagli M., Susluoglu Z. Micropropagation of mature Crataegus aronia L., a medicinal and ornamental plant with rootstock potential for pome fruit // Plant Growth Regul. - 2012. - V. 67. - P. 57-63. |
| [15] | Bell, R.L. In vitro tissue culture of pear: Advances in techniques for micropropagation and germplasm preservation / R.L. Bell, B.M. Reed // Proc. 8th IS on Pear. – 2002. – P. 412-418. |
| [16] | Butenko R.G. Biology of cells of high plants invitroand biotechnology on their basement: teaching manual // Мoscow (UBK-Press), 1999. - Р.160. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




