-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2021; 9(1): 16-20
doi:10.5923/j.ijge.20210901.03
Received: Mar. 15, 2021; Accepted: Apr. 7, 2021; Published: May 15, 2021

Regeneration of Sorghum through Tissue Culture Techniques
Rasha Adam Omer, Sufian Suliman, Mayada Mamoun Beshir
Biotechnology and Biosafety Research Center, Agricultural Research Corporation, Shambat, Sudan
Correspondence to: Rasha Adam Omer, Biotechnology and Biosafety Research Center, Agricultural Research Corporation, Shambat, Sudan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This is the first study in East and Central Africa for optimization tissue culture protocol of sorghum. The effect of hormones on callus induction of sorghum and finally to assess the regeneration response of sorghum through tissue culture techniques. Callus induction and regeneration of four sorghum varieties (Tabat, Elwafer, Yrwasha and Dwarf white - Mylo) was evaluated using mature embryos as source of explants and different concentration of the hormone 2,4-D (0, 1, 2, 4, 6, mg/L) supplemented with 0.2 mg/L Kinetin. The highest callus induction frequency was observed in 2 mg/L for Elwafer and D.W. Milo while 6 mg/L of 2,4-D level gave higher callus induction frequency for Tabat and Yrwasha. The lowest callus induction frequency was observed in 0 and 1 mg/L of 2,4-D level for all the varieties. The highest embryogenic callus induction frequency was observed in Tabat at 4 mg/L and 6 mg/L of 2,4-D while the lowest embryogenic callus induction frequency was observed in 0 and 1mg/L of 2,4-D. Regeneration efficiency was observed higher in the variety Tabat at 4 mg/L of 2,4-D level.
Keywords: 2,4,D, Sorghum, Mature embryos, Callus
Cite this paper: Rasha Adam Omer, Sufian Suliman, Mayada Mamoun Beshir, Regeneration of Sorghum through Tissue Culture Techniques, International Journal of Genetic Engineering, Vol. 9 No. 1, 2021, pp. 16-20. doi: 10.5923/j.ijge.20210901.03.
Article Outline
1. Introduction
- Sorghum (Sorghum bicolor L.) it is the fifth important cereal crop grown in the world (US Grains Council). In the years 2012/2013, production of sorghum was reported to be 59.3 million metric tons worldwide (USDA Grain: World Markets and Trade, Feb. 2012/2013).Sorghum used as staple food for over 500 million people in the world, especially in African and Asian countries. Sorghum is rich in antioxidants and it is grain free of gluten and for this reason provides an attractive grain replacement for people suffer from celiac disease. Sorghum is drought-resistant crop addresses global climate change and limited water resources issues, sorghum used as source of food, feed and fiber, is also (Kargi et al., 1985; Gnansounou et al., 2005; Laopaiboon et al., 2007; Rooney et al., 2007). There are several reports indicating the tolerance of sorghum to drought and high temperature than corn, wheat and other cereal crops (Gnansounou et al., 2005). Sorghum has the unique ability to grow under a wide range of harsh environmental conditions (House, 1995).Modifications of tissue culture protocols of sorghum have been reported (2010). In these reports, based on callus induction experiments, the highest callus induction reported were 4.5% (Shridhar et al., 2010). Transformation of sorghum via Agrobacterium-mediated method was reported unsuccessful due to it is recalcitrant for tissue culture and genetic engineering (Shrawat and Lorz, 2006). Regeneration of transgenic sorghum was successfully reported using Agrobacterium-mediated transformation (Zhao et al., 2000). This was followed by reports from other researchers using Agrobacterium-mediated transformation method (Gurel et al., 2009; Howeet et al., 2006; Lu et al., 2009; Nguyen et al., 2007). There are several factors that affect transformation efficiency, including the sensitivity of sorghum explants to Agrobacterium infection, type of explant, composition of the culture media. Addition of coconut water to the co-cultivation medium together with the use of vigorous and actively growing immature embryos as explants for infection and the removal of excess Agrobacterium significantly improved the survival rate of explants and were critical for the success of transformation (Hiei et al., 1997).
2. Material and Methods
- Plant materialsSeeds of four sorghum varieties (Tabat, Elwafer, Yrwasha and Dwarf White Milo (D.W. Milo)) were sterilized by first soaking in 70% ethanol for 30 seconds. The seeds were then soaked in 2.5% sodium hypochlorite for one hour before washing with sterile water three times under sterilized conditions. The sterile seeds were used as sources of mature embryos as explants for callus induction.Callus induction and culture conditionsAll callus induction experiments were performed on callus induction medium (CIM). The CIM comprised of MS salts and vitamins supplemented with 0.7 g/l L-Proline, 0.5 g/l MES, 0.5 g/l casein hydrolysate, 0.2 mg/L Kinetin and 3% (w/v) sucrose. The pH of the medium was adjusted to 5.8 with 1M NaOH or 0.1M HCL. 0.65% (w/v) gelrite was added to solidify the medium. CIM was sterilized by autoclaving. Five levels of 2,4-D (0, 1, 2, 4, and 6 mg/l) were tested to establish their efficacy in establishing callus from four different varieties of sorghum via mature embryos. The Mature embryo explants were obtained from sterile seeds washed in distilled and autoclaved water. Mature embryos were cultured using a sterile forceps on CIM containing different 2,4-D levels (0, 1, 2, 4, 6, mg/L). The embryos were orientated with the embryo axis in contact with the medium. Twenty embryos were cultured in a 90 x 15 mm Petri dish for each 2,4-D level. The cultures were completely covered with aluminium foil and incubated in the dark at 25±1°C for two weeks. Callus induction frequency was recorded after four weeks of culture on the CIM.After six weeks of callus induction all calli were transferred to MS medium for 14 days to initiate shoots. For induction of shoots the embryogenic calli were transferred to shoot induction medium (RII) comprising MS basal salts and vitamins (Murashige and Skoog, 1962), supplemented with 30 g/L sucrose, casein hydrolysate and proline. The number of regenerated plantlets per calli was evaluated.Statistical analysisThe experiments were designed in randomized complete block design (RCBD) with four replications per treatment. Callus induction frequency were calculated on number callus induced from the total number of explants were cultured, these data were used to compute callus induction frequency. Analyses of variance (ANOVA) were done by using statview statistical software to test the statistical significance of differences among explants source and 2,4-D levels. Mean separation was done using least significance difference (LSD) test at 5% probability level.
3. Results and Discussion
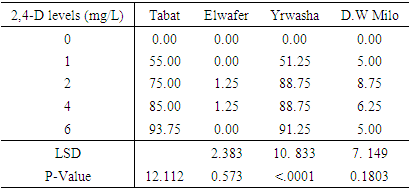
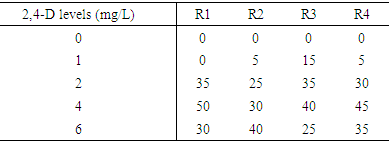
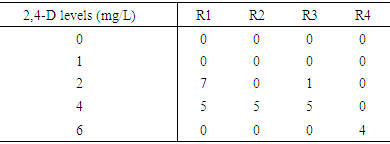
- Clear differences in callus induction frequencies were observed among the four varieties used in this project, the variety Elwafer gave the lowest callus induction frequency among all the varieties. While the variety Tabat gave the highest callus induction frequency among all the varieties. Callus induction frequency from variety Tabat was highest at 2,4-D concentrations of 4 and 6 mg/L with callus induction frequencies of 85.0 and 93.8 respectively (Table 1). This was significantly higher than at other concentrations but there was no significant difference in callus induction between the concentrations 2 and 4 mg/L (Figure 2C). There was no significant difference in callus induction frequency for all the treatments for the variety Elwafer the concentrations 2 and 4 mg/L gave the same and the highest callus induction (1.250 and 1.250) (Table 1) with no significant differences between them. The variety Yrwasha gave the highest callus induction frequency at concentrations 2, 4 and 6 mg/L of 2,4-D and there were no significant differences in callus induction for those three concentrations but there were significant differences between the other 2,4-D level (Figure 2B). For the variety Dwarf Milo the highest callus induction frequency was obtained at 2,4-D concentration of 2 and 4mg/L with means of 8.750 and 6.250. This was not significant than that of other 2,4-D concentrations but there was significant differences between 0 mg/Land 2 mg/L of 2,4-D (Table 1).
|
|
|
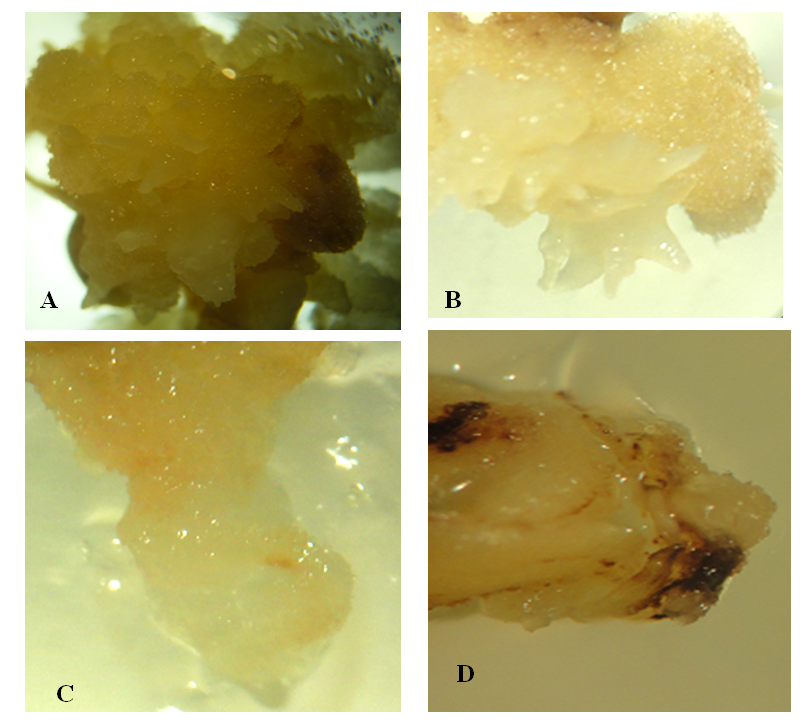
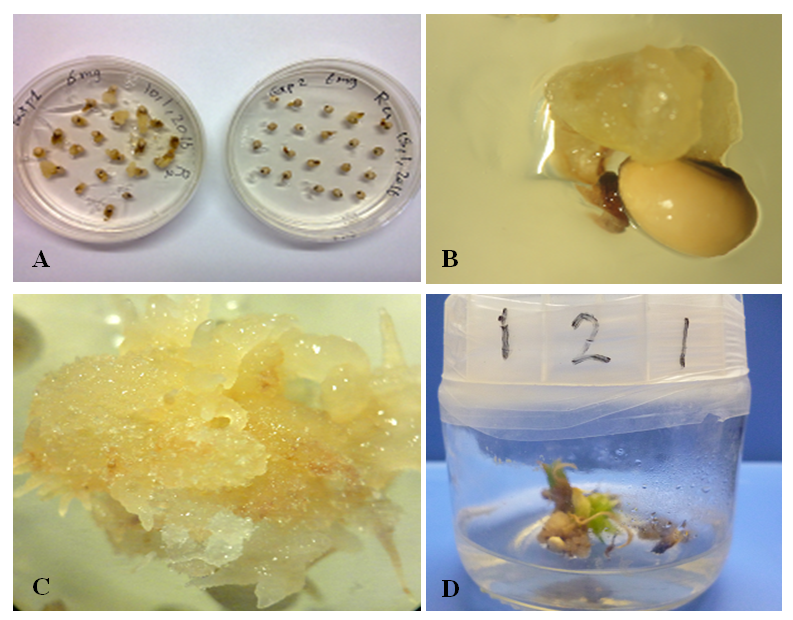 | Figure 2. Callus induction and regeneration from mature embryos of sorghum achieved using different concentrations of 2,4,D |
4. Conclusions
- This work has formed the base for transformation of sorghum there for transformation can be achieved because we have the infrastructure and protocols for sorghum at Agricultural Research Corporation of Sudan.The variety Tabat it is the most promising sorghum genotype and shall be used for tissue culture and transformation of sorghum.Tissue culture research has been optimized in sorghum there for protocols for tissue culture of millet and finger millet can be establish for enhancement of cereal crops.
ACKNOWLEDGEMENTS
- This work was achieved through funding provided by African Bioscience Challenge Fund Fellowship, the work was carried out in the Tissue Culture Unit in the Biotechnology and Biosaftey Research Center at agricultural research corporation of Sudan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML