-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2017; 5(1): 1-10
doi:10.5923/j.ijge.20170501.01

Breeding Thai Rice for Flood Tolerance through Electron Beam-induced Mutations
Udompan Promnart1, Vichai Puripunyavanich2, Kanokporn Boonsirichai2, Peera Doungsoongnern1, Ruenruedee Kaewchuenchai1, Surachet Chamotri3, Kanchana Klakhaeng3, Fatma Sarsu4
1Prachin Buri Rice Research Center, Bansang, Prachinburi, Thailand
2Thailand Institute of Nuclear Technology, Ongkharak, Nakhon Nayok, Thailand
3Rice Department, Thailand
4International Atomic Energy Agency
Correspondence to: Kanchana Klakhaeng, Rice Department, Thailand.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

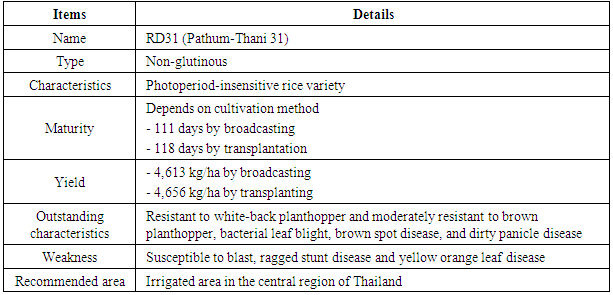
Rice is a staple food for Thailand and other Asian countries. Recently, flooding caused by the climate change has had a severe effect on the rice production in Thailand. Hence, breeding Thai rice for flood tolerance has become more important and an agenda for the Thai researchers. One of the breeding methods is using radiation to generate the genetic variation. Standard electron beam irradiation was mapped on 23 Thai rice varieties. RD31, a non-glutinous, photoperiod-insensitive rice variety, was selected after the irradiation due to its several preferable agricultural traits, such as broad adaptation for diverse environments, resistance to white-back planthopper and high yield. The RD31 seeds were irradiated with 0.44 kGy electron beam. Then, the M1 plants were planted in January 2014 and 500 main panicles were selected and harvested. After that, the M2 plants were planted in December 2015. Agricultural traits, such as plant height and maturity date were observed in these plants. After that, about 3000 M3 plants were selected and screened for submergence tolerance, and 317 tolerant mutant lines were identified. Furthermore, screening for blast resistance and good grain quality was carried out in the M4 and M5 generations to select for more traits.
Keywords: Rice, Electron Beam, Radiation Mutagenesis, Breeding, Flood Tolerance
Cite this paper: Udompan Promnart, Vichai Puripunyavanich, Kanokporn Boonsirichai, Peera Doungsoongnern, Ruenruedee Kaewchuenchai, Surachet Chamotri, Kanchana Klakhaeng, Fatma Sarsu, Breeding Thai Rice for Flood Tolerance through Electron Beam-induced Mutations, International Journal of Genetic Engineering, Vol. 5 No. 1, 2017, pp. 1-10. doi: 10.5923/j.ijge.20170501.01.
Article Outline
1. Introduction
- Thailand has a land area of 513,115 square kilometers. The country is located in the tropical area. There are three seasons: summer from March to May, rainy season from June to October, and winter from November to February. Temperature normally varies between 19°C and 38°C with an average of 29°C. The humidity ranges from 66% to 83%. Thailand with it tropical climate has become a major rice producer and exporter of the world with its cultivation area covering approximately 437 million hectares and exporting about 9.9 million tons of rice in 2016 (Office of Agricultural Economics, 2015).In Thailand, rice is grown in diverse ecosystems which could be divided into four ecosystems, including 39.5 million hectares of rainfed lowland rice, 7.69 million hectares of irrigated lowland rice, 2.05 million hectares of upland rice and 15.5 million hectares of deep water rice (Hydro and Agro Informatics Institute, 2013). Rainfed and irrigated lowland areas in Chao Praya basin are important rice production areas and named the rice bowl of Thailand. The areas cover lower northern plains in Phichit, Phitsanulok and Nakhon Sawan province as well as central plain in Suphan Buri, Phranakhon Si Ayutthaya, Pathum Thani and Nonthaburi province with the approximate area of 2.4 million hectares (Hydro and Agro Informatics Institute, 2013). These areas often encounter recurrent and seasonal floods and those floods become more severe with faster flow rate and larger volume of water during the major cropping season between August and November due to the climate change.There are two types of flood. The first one is deep water flood which features a long period of deep water flooding. The second one is flash flood which features a short period and the water level is not deep (Nagai et al., 2010). While deep water flood is fairly predictable, the flash flood is extremely unpredictable and may occur at any stage of rice growth. The great flood in 2011 was the worst flash flood in 50 years in Thailand and had a great impact on on rice production in Chao Praya basin (IAEA, 2012). At least 25 out of 77 provinces of the country were heavily affected. More than 9 million people were evacuated. At that time, most cultivated area in the central plain were submerged. The reduced oxygen and carbon dioxide availability caused the huge yield loss in rice production. Thus, submergence-tolerant rice is highly desirable and expected to enhance food security for Thai people as well as the world population.The major morphological and physiological traits for submergence tolerance are slow leaf elongation, less chlorosis and high carbohydrate reserve storage during submergence and prompt re-adaptation to the aerial environment after de-submergence (Setter et al., 1997; Ito et al., 1999; Ram et al., 2002; Jackson and Ram, 2003). Regarding to the traits above, carbohydrate metabolism during submergence is likely the key factor for flash-flood tolerance, and this ‘quiescence strategy’ is characterized by slow expansion growth, presumably, to conserve energy and carbohydrates (Singh et al., 2001). The rapid shoot and leaf elongation is adversely affected by submergence (Jackson et al., 1987; Setter and Laureles, 1996; Ito et al., 1999; Kawano et al., 2002; Ram et al., 2002; Jackson and Ram, 2003). Generally, in plant genetics and breeding research, mutation serves as a source of variation. As spontaneous mutation rate is very low, induced mutation has contributed to the discovery and identification of gene function in the past decades (Liang, 2009). Radiation serves as an effective agent to induce mutations or genetic changes in living organisms at a much higher frequency than spontaneous events (Kharkwal and Shu, 2009). Furthermore, mutation breeding can potentially lead to sustainable agriculture by providing new varieties with novel agricultural traits, especially in cereals, legumes, fruits, vegetables, fiber and oil crops (Liang, 2009; Kharkwal and Shu, 2009). In order to cope with recurrent seasonal flash floods, therefore, radiation-induced mutagenesis in rice is a promising solution. Submergent-tolerant mutants can be identified and developed into a cultivar which could be later adopted by farmers in the target regions.The objective of this study was to explore the potential of 8 MeV electron beam in radiation-induced mutagenesis in order to improve RD31 cultivar for submergence tolerance and resistance to important diseases and insects with high yield and good grain quality.
2. Materials and Methods
2.1. Sensitivity of Rice Cultivars to Electron Beam
- Seeds from 23 Thai rice cultivars (Table 3) and one foreign cultivar (Swarna-Sub1) were obtained from Prachin Buri Rice Research Center and Pathumthani Rice Research Center, Thailand. For radiation treatments, 100 seeds of each cultivar were irradiated with 0 - 1,200 Gy electron beam at Gems Irradiation Center, Thailand Institute of Nuclear Technology, Thailand. The beam had an energy of 8 MeV, a current of 100 mA, a scaling dose of 1.18 k Gy m/min and a maximum pulse repetition frequency of 10 – 120 Hz. Then, 3 replicates of irradiated seeds were germinated on moist tissue paper in a plastic box and kept in darkness. Their germination rates were recorded for 7-day post-irradiation. To calculate the germination rate, unirradiated seeds were used to normalize the germination rates with radiation treatments. Comparisons between treatments were statistically analyzed by using Student’s t-test. A p-value of less than 0.05 was considered significant.
|
2.2. Isolation of Submergence-Tolerant Mutants
- RD31 was a promising cultivar for mutation breeding because it had several preferable agricultural traits as shown in Table 1. The seeds were supplied by Prachin Buri Rice Research Center, Rice Department, Thailand. About 200 g of seeds were irradiated with 440 Gyelectron beam. Then, the irradiated seeds were germinated. After that, the M1 plants were plated as one plant/hill, and 500 panicles from the main tiller were collected to obtain M2 seeds. Then, M2 plants were planted as panicle/row for 500 rows or 10,000 plants in total. Seeds from six plants in each row were collected as M3 seeds. Agricultural traits, including plant height, number of panicle per hill and maturity of these M2 plants were observed and recorded. Then they were screened for submergence tolerance.
|
2.3. Screening for Submergence Tolerance
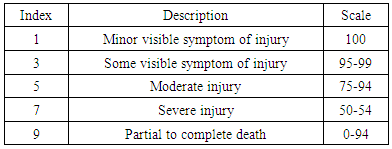
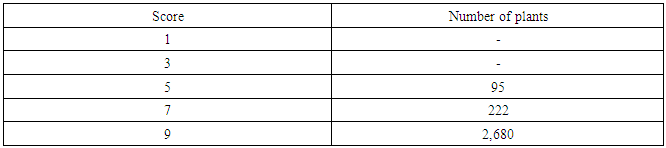
- About 30 g of seeds from each 3,000 M3 lines were germinated and subsequently planted in the row in the experimental field. The row of each M3 line was 75 cm long and 25 cm wide. The FR13A, tolerant control, the IR42, susceptible control, and the parental RD31 were planted after every tenth M3 row for comparison.Fourteen days after germination, water level was raised to 10 cm (Figure 1A). In the 30th day, the water level was suddenly increased to 150 cm and maintained for 14 days as a flash flood (Figure 1B). The M3 lines were observed once a week for their performance under flooding condition. After 14 days of flooding, the water was drained out (Figure 1C), to allow the plants to recover for 14 days. Then, visual scoring for recovering ability was carried out according to Standard Evaluation System for Rice (IRRI, 1996) (Table 2). The tolerant individuals remained alive and recovered, while the susceptible individuals died under the flood or failed to recover. Seeds were collected from the tolerant plants for planting in the following seasons.
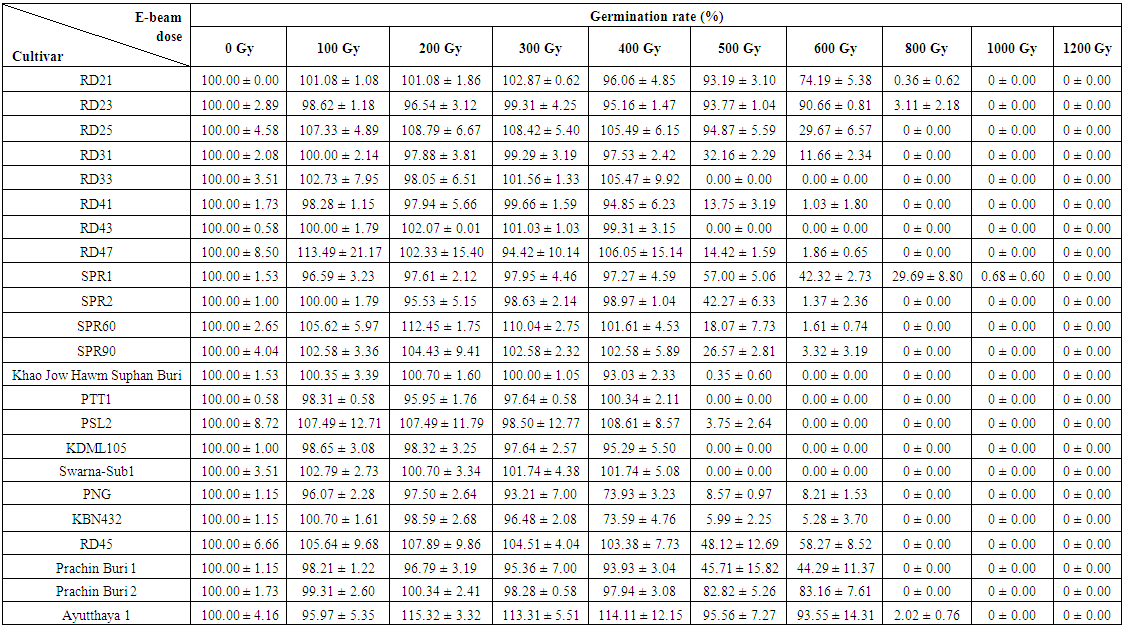 | Table 3. Effects of electron beam irradiation on the germination rate (mean±SD) of Thai rice cultivars |
 | Figure 1. Screening for submergence-tolerant mutants: (A) before submergence, (B) during submergence and (C) after submergence |
3. Results
3.1. Effect of Increasing Electron-Beam Doses on Seed Germination
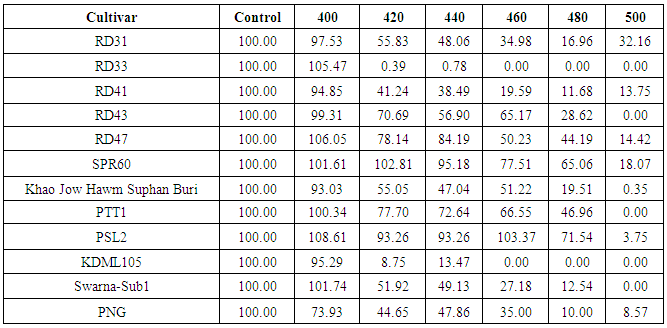
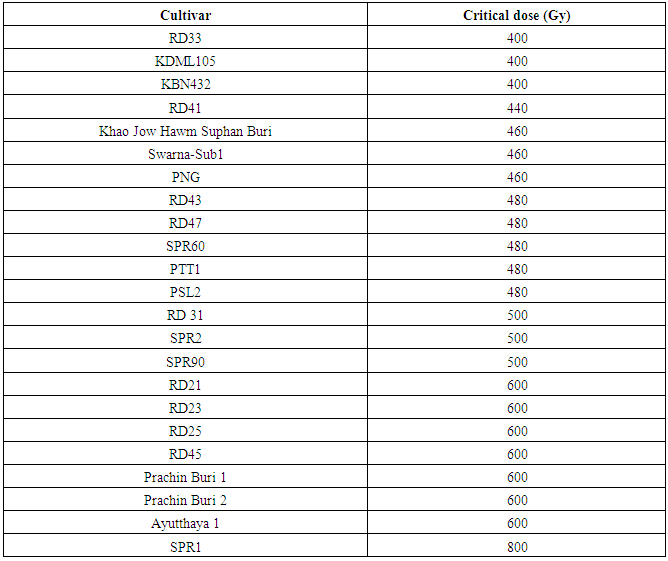
- Seeds from 23 cultivars were irradiated with 8 MeV electron-beam at 100 Gy intervals from zero to 1,200 Gy. Germination rates were recorded seven days after seeding. The germination rates of 23 cultivars were not significantly affected by the electron-beam up to 300 Gy (Figure 2). Surprisingly, for some cultivars including, RD21, RD25, RD43 and SPR60, an electron-beam dose of up to 300 Gy showed a positive effect on their seed germination rates (p< 0.05). At 400 Gy, two cultivars, including Khao Bahn Nah 432 (KBN432) and Plai Ngahm Prachin Buri (PNG), showed over 10% reduction in seed germination rates. Most rice cultivars experienced a sharp drop, even more than 40% in their germination rate after receiving a 400 Gy dose. However, Prachin Buri 2, Ayutthaya 1, RD21 and RD23 were among the most tolerant cultivars to electron-beam radiation with a sharp drop in germination rate observed only after 600 Gy irradiation.LD50 is the dose at which the germination rate of irradiated population reduces by 50%, and is often regarded as the suitable dose for mutation induction in a breeding program. An LD50 is usually determined by plotting the dose response curve with a linear or log-linear function and calculating the 50% survival dose. From our dose response data, an LD50 could not be identified for most cultivars. Instead of showing a gradual, linear decrease in germination rate with an increasing dose, all cultivars showed a sharp drop of 40% or more after receiving a certain dose of electron beam, which reduced the germination rate sharply to almost zero in half of the tested cultivars (Figure 2). LD100 is however defined as the minimum radiation dose that could cause 100 percent reduction in seed germination rate. KDML105, PTT1, RD33, RD43 and Swarna-Sub1 were among the most sensitive cultivars with the LD100 at 500 Gy (Table 5). Interestingly, SPR1 had the highest LD100, which was 1,200 Gy. Other cultivars that also had a high LD100 included Ayutthaya1, RD21 and RD23 (Table 5; Figure 2).
3.2. Critical Dose Measurement
- As stated earlier, LD50 could not be determined for most rice cultivars treated with electron-beam. We proposed to characterize the germination response to electron-beam irradiation using the term ‘critical dose’. The critical dose was identified as the maximum dose before the observed germination rates declined by 20%. The germination rate of rice seeds failed to show a linear or log-linear dose response curve against varying doses of electron beam. Each observed cultivar had its own critical dose. If the seeds were exposed over their critical dose, their germination rates would be severely reduced to almost zero. For example, RD21 showed a critical dose of approximately 600 Gy with 74.19% relative germination rate. A dose of 800 Gy, however reduced its germination rate to below 1%. Similarly, RD23 cultivar with a critical dose of approximately 600Gy also had a 90.66% relative germination rate. However, its germination rate was reduced to 3.11% at 800 Gy (Table 3).In addition, SPR1 showed the highest critical dose, approximately at 800 Gy, with 29.69% germination rate. When irradiated with a 1,000 Gy dose, its seeds almost completely failed to germinate. Similarly, SPR2 had a critical dose of 500Gy with a germination rate of 42.27%. Exposure of SPR2 to 600Gy electron beam, however decreased its germination rate to 1.37% (Table 3). In contrast to SPR1, RD33, KDML105 and KBN432 showed the lowest observed critical dose around 400 Gy, while RD21, RD23, Prachin Buri 1, Prachin Buri 2 and Ayutthaya 1 showed the highest observed critical doses at 600 Gy.Germination rates of the cultivars in response to 400 – 500 Gy electron beam were studied in detail at 20 Gy intervals (Table 4). Their germination rates were also recorded 7-day post-irradiation. Six of the irradiated Thai rice cultivars, including RD31, RD41, RD43, RD47, Swarna-Sub1and PNG showed a linear response to radiation and the other five cultivars, including RD33, SPR60, Khao Jow Hawm Suphan Buri, PTT1, PSL2 and KDML105 still showed a response curve with the critical dose. Interestingly, KDML105 had a critical dose of 400 Gy with 95.29% germination rate. A little higher exposure, at 420 Gy however, decreased its germination rate sharply to 8.75% (Table 4).
3.3. Isolation of Submergent-Tolerant Mutants of RD31 Cultivar
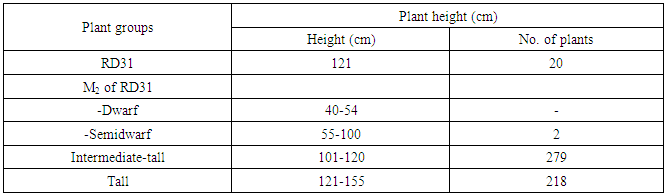
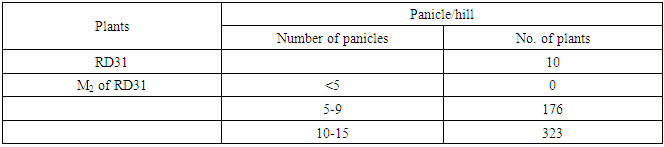
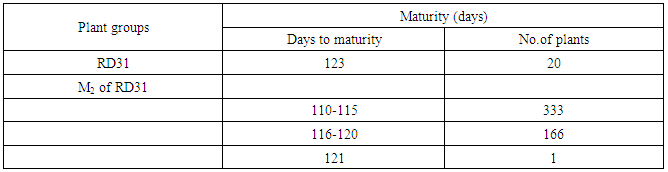
- RD31, a non-glutinous and photoperiod-insensitive rice cultivar, is one of the recommended cultivars for irrigated areas in the central plain of Thailand. It has a relatively high yield and moderate resistance to brown planthopper, bacterial leaf blight, brown spot disease and dirty panicle disease with a good grain quality.M1 plants were planted in dry season 2014. Five hundred panicles were harvested from the main tiller of each M1 plant to obtain M2 seeds. Then, M2 plants were also planted in dry season 2014 (Figure 3). Plant height, number of panicle per hill and maturity of these M2 plants were observed and recorded (Table 6).The plant height of RD31 was 121 cm. It was classified according to Loresto and Chang (1978) as tall class. Upon electron-beam irradiation, the height of M2 plants ranged from 99-143 cm (Table 6 and Figure 4), covering all three height classes, including semi-dwarf, intermediate-tall and tall. The observed frequencies were 2, 279 and 218, respectively.Panicle number of each hill was counted as it was a key component of the yield. Yield components were determined during each rice cycles, first at the vegetative phase (tiller number), second at the reproductive phase (number of fertile tillers and size of panicle sinks) and finally at the grain filling phase (spikelet filling rate). The results showed that M2 population provided as 5-9 panicle/hill in 176 plants and 10-15 panicle/hill in 323 plants (Table 7). Normally, maturity of RD31 depends on planting method which is 118 days for transplanting and 111 days for broadcasting. The M2 plants were grown by transplanting and the maturity dates were varying. Approximately 500 M2 plants with shortened maturity period were selected. Three hundred and thirty-three of them had 110-115-days for maturity; 166 plants with 116-120 days; one plant with 121 days (Table 8). Shortened maturity period was the preferable trait to cope with climate change. The central plain of Thailand frequently encounters a water shortage during the dry season which is followed by a heavy flood during the rainy season. Cultivars with a shortened maturity period are highly desirable because harvesting can be done early before the flooding period. Therefore, two crops will be possible per year before and after the flooding period. About 3,000 M3 lines were screened for submergence tolerance. The plants were submerged under a flash flood condition for 14 days. Only 317 lines survived the flood with the score of 5-7, which were selected for planting in the following season and further analysis. (Table 9, Figure 5 and 6).
|
|
|
|
|
|
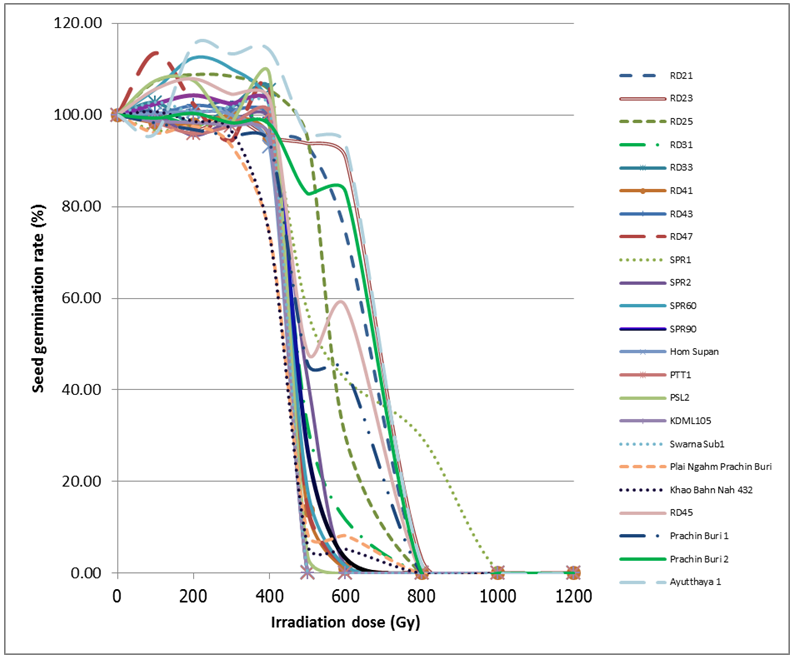 | Figure 2. The effects of electron beam irradiation on the germination rate of Thai rice cultivars; germination rates at zero Gy were adjusted to 100% |
 | Figure 3. Electron-beam irradiated M2 plants of RD31 |
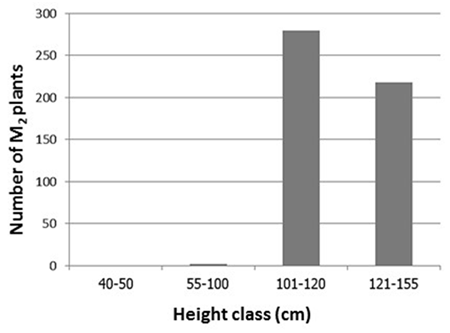 | Figure 4. Distribution of M2 plant height according to the classification of Loresto and Chang, (1978) |
 | Figure 5. Submergence-tolerant mutant lines from field screening |
 | Figure 6. Protection of 317 mutant lines from bird damage by nets |
4. Discussion
- Although electron beam is less understood, its mutagenic effects were shown for unicellular organisms, including budding yeast Saccharomyces cerevisiae (Zhu et al., 2008; Zhang et al., 2012) and microalgae Arthrospira platensis (Kim et al., 2013). In plants, 2 MeV of electron beam successfully induced mutations in Arabidopsis thaliana causing the same classes of DNA aberrations as seen with C6+ ions (Shikazono et al., 2005). In yeast cells, electron beam radiation had little influence on the function of plasma membrane and protein, while it could induce DNA damages of single strand breaks (SSB) and double strand breaks (DSB) that were required for gene mutation. The G-value for DSB formation of electron beam radiation in aqueous solution was 5.7 times higher than that caused by 60Co gamma rays (Zhu et al., 2008). In this study, it has presented that rice cultivars had a varying degree of sensitivity to electron beam. Among 23 cultivars that were irradiated, KDML105 and RD33 were among the most sensitive cultivars, while Ayutthaya 1, RD21, RD23 and SPR1 were among the most tolerant to the radiation considering their germination rates. RD31, one of the recommended cultivar which was chosen for mutation breeding was irradiated with 440 Gy electron beam.In fact, electron beam in mutation breeding has been a potential technique to generate variation for breeding since 1930s. This method has been widely applied on several plant species, including cereals, legumes, fruits, vegetables, fiber and oil crops (Bamidele and Akanbi, 2013). Moreover, it has also been applied on rice (Oryza sativa L.) to generate variation to improve grain yield and quality and the promising results were obtained (Ibrahim, 2013). In this study, it has demonstrated that electron beam in radiation-induced mutagenesis can contribute to a breeding program for submergence-tolerant rice.
4.1. Isolation of Submergent-Tolerant Mutants Based on Physiological Characters
- IRRI released the first high-yielding modern rice cultivar, the IR8, for the tropical irrigated lowlands in 1966 (Chandler, 1969). Since then, IRRI has developed many high-yielding rice varieties such as IR36, IR60 and IR72 (Peng and Khush, 2003). New plant type (NPT) was developed at IRRI in the late 1980s. The NPT was designed based on the results of simulation modeling. The proposed NPT has a low tilling, few unproductive tillers, 200-250 grains per panicle, a plant height of 90-100 cm, thick and sturdy clumps, dark green and erect leaves, vigorous root system with 100-130 days for maturity (Peng et al., 1994).Under flooding condition, the oxygen and carbon dioxide level are reduced. Photosynthesis and cellular respiration are also affected. Xu and Mackill (1996) reported a major gene that controlled the ability of rice to tolerate submergence condition, which was named Sub-1. Then, several studies have shown that plants with sub-1 gene could survive under a fully submerged condition for two weeks. Quantitative trait locus (QTL) analysis revealed that submergence tolerance is controlled by a single major QTL on chromosome 9 along with a number of other minor QTLs (Xu and Mackill, 1996; Nandi et al., 1997; Toojindaet al., 2003).Approximately 3,000 M3 lines were screened in this study and 317 submergence tolerant lines were identified and selected. Further work will be carried out in M4 and M5 generations to select for more preferable traits.
ACKNOWLEDGMENTS
- The authors would like to thank IAEA for technical cooperation under THA5054. The project was funded by Rice Department of Thailand and Thailand Institute of Nuclear Technology. Technical assistance on germination response to electron beam was provided by Vararas Khamvarn.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML