-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2013; 3(1): 1-5
doi:10.5923/j.ijge.20130301.01
Stromal Cell-derived Factor (SDF-1 β) Gene Single Nucleotide Polymorphism at Position G801A is Associated with Type 2 Diabetes Mellitus in a South Indian Population
Dhamodharan Umapathy1, Ezhilarasi Krishnamoorthy1, Parthiban Muthukumaran1, Rama Rajaram2, Indira Padmalayam3, Vijay Viswanathan1
1Department of Biochemistry & Molecular Genetics, Prof.M.Viswanathan Diabetes Research Centre & M.V.Hospital for Diabetes (A WHO Collaborating Centre for Research, Education & Training in Diabetes), Royapuram, Chennai, 600 013, India
2Department of Biochemistry & Biomaterials, Central Leather Research Institute, Adyar, Chennai, 600 020, India
3Drug Discovery Division, Southern Research Institute, Birmingham, Alabama, 35205, USA
Correspondence to: Vijay Viswanathan, Department of Biochemistry & Molecular Genetics, Prof.M.Viswanathan Diabetes Research Centre & M.V.Hospital for Diabetes (A WHO Collaborating Centre for Research, Education & Training in Diabetes), Royapuram, Chennai, 600 013, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
In this case-control study, we investigated the association of G801A single nucleotide polymorphisms (SNPs) in the SDF-1β gene (SDF-1β 3’A) with type 2 diabetes (T2DM) in a south Indian population. In this assessment, peripheral blood samples were collected from 150 T2DM patients and 150 healthy controls who attended our tertiary care hospital in Chennai, India. Peripheral blood genomic DNA was extracted and subjected to PCR-RFLP, to examine SDF-1β gene polymorphism. Our results showed that the genotype frequency of “GG” was significantly associated with T2DM when compared to healthy controls (P <0.001). Interestingly, our results also showed that SDF-1 “G” allele to be risk allele [1.238 (0.899-1.706)] and “A” to be the protective allele for T2DM at least in our south Indian population. Based on the results of this study, we conclude that SDF-1β 3’A polymorphism to play a role in the pathogenesis of T2DM.
Keywords: SDF-1β, Type 2 diabetes, SNPs, Genotypes
Cite this paper: Dhamodharan Umapathy, Ezhilarasi Krishnamoorthy, Parthiban Muthukumaran, Rama Rajaram, Indira Padmalayam, Vijay Viswanathan, Stromal Cell-derived Factor (SDF-1 β) Gene Single Nucleotide Polymorphism at Position G801A is Associated with Type 2 Diabetes Mellitus in a South Indian Population, International Journal of Genetic Engineering, Vol. 3 No. 1, 2013, pp. 1-5. doi: 10.5923/j.ijge.20130301.01.
Article Outline
1. Introduction
- According to the diabetes atlas 2011, the number of people living with diabetes is expected to rise from 366 million in 2011 to 552 million by 2030. India, the world’s second most populous country, now has more people (61.3 million) living with type 2 diabetes (T2DM); this places India second to China. T2DM is considered a multifactorial pathology that involves insulin resistance and is associated to obesity, dyslipidemia, endothelial dysfunction and inflammation[1]. Although T2DM has a strong genetic basis, until recently, most candidate genes for T2DM have shown only modest effects and the associations have been inconsistent[2,3]. Cytokines are a group of pharmacologically active low molecular weight proteins that possess autocrine and paracrine effects[4] and are known products and effectors of the inflammatory and immune system[5].They have recently been the focus of several studies due to their crucial roles in T2DM and its complications and the impact of cytokine imbalance in late diabetic complications has been reported earlier. In T2DM, peripheral blood monocytes express more inflammatory cytokines than those from healthy subjects[6]. Cytokines have several biological effects such as recruitment of leukocytes to the sites of injury/infection and inflammation, angiogenesis, and angiostatic properties[7]. According to the presence of cysteine residues in their structure, they are classified as CC, CX3C, C, and CXC[8]. Stromal cell– derived factor (SDF)-1/CXCL12 is a peptide chemokine initially identified in bone marrow–derived stromal cells and now recognized to be expressed in stromal tissues in multiple organs[9,11]. SDF-1 is located on chromosome 10q 11.1[12]. The 2 isoforms, SDF-1α and SDF-1β, arise from a single gene by alternative splicing [13]. Sequence analysis reveals a common polymorphism in the 3’-untranslated region, implicated in mRNA turnover regulation, of the SDF-1β gene transcript, which contains a G to A transition at position 801, designated SDF1-3’UTR-801G-A, abbreviated as SDF1-3’A [14]. It has been reported that SDF1-3’A genotype action involves up-regulation of the quantity of SDF-1 protein available to bind CXCR4[13]. The SNP of SDF-1 G801A has been investigated in various diseases such as: type 1 diabetes[15] HIV-1 infection[16], inflammatory diseases[17] lymphoma [18] and cancers[19]. Therefore the aim of the study was to investigate the functional SNP of the SDF-1β gene in a south Indian population with T2DM.
2. Methodology
2.1. Study Participants and Measurements
- Peripheral blood samples were collected from 150 unrelated T2DM patients attending outpatient department of M.V.Hospital for Diabetes, Chennai, India and 150 healthy controls (mostly blood donors and hospital staffs). Both patients and controls were of same ethnic origin. Patients had also similar disease severity because they were not suffering from any other diabetes complications. Anthropometric and certain biochemical parameters were recorded. Biochemical analyses were done on a Hitachi-912 Autoanalyzer (Hitachi, Mannheim, Germany) using kits supplied by Roche Diagnostics (Mannheim, Germany). Fasting plasma glucose (glucose oxidase-peroxidase [GOD-POD] method), serum cholesterol (cholesterol oxidase-phenol4-amino antipyrene peroxidase [CHOD-PAP] method), serum triglycerides (glycerol phosphatase oxidase−phenol4-amino antipyrene peroxidase [GPO-PAP] method), and high-density lipoprotein cholesterol (direct method–polyethylene glycol-pretreated enzymes) were measured. Low-density lipoprotein cholesterol were estimated directly by homogenous method using diasys kit protocol. Glycosylated hemoglobin (HbA1c) was estimated by high performance liquid chromatography using the Turbo Variant machine (Bio-Rad, Hercules, CA). Serum creatinine was measured using the Jaffe method. The study protocol was approved by the institutional ethical committee of M.V.Hospital for Diabetes. Prior to sample collection, all participants of this study filled out and signed the informed consent form.
2.2. DNA Extraction and Genotyping
- Genomic DNA was extracted from whole blood by proteinase K digestion followed by phenol-chloroform extraction[20]. Extracted DNA was aliquoted for each sample and stored at -20°C for further analysis. The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was used to genotype the G801A polymorphism in the SDF-1β gene, as described elsewhere [21]. Genotyping was carried out using the following primers: Forward 5’-CAG TCA ACC TGG GCA AAG CC-3’ and Reverse 5’-AGC TTT GGT CCT GAG AGT CC-3’(Sigma, Bangalore, India). The reaction mix for polymerase chain reaction contained 50 ng of DNA, 10 pmol of oligonucleotides and a 2x solution of Prime Taq Premix (Genet Bio, Chungnam, Korea). The PCR cycle began with an initial denaturation step at 94°C for 5 mins, followed by 35 cycles of denaturation at 94°C for 30 secs, annealing at 58°C for 30 secs, and extension at 72°C for 2 min followed by a final extension at 72°C for 5 min. After PCR, 10 μL of the reaction mixture was digested with 5U of MspI restriction endonuclease (Fermentas, Germany) in a 20μL reaction volume at 37°C for 2 hrs. The digested products were electrophoresed on a 2.0% agarose gel after staining with ethidium bromide, visualized on a Gel-Doc model XR+ (Bio-Rad, USA). Size was determined by comparison to a molecular weight standard 1 kb plus (Invitrogen, Carlsbad, CA).
2.3. Statistical Analysis
- Hardy-Weinberg equilibrium was assessed using genotype data. Allele and genotype frequencies were calculated in patients and healthy controls by direct gene counting. Statistical analysis of the differences between groups was determined by χ2 test and SPSS software version 10. Relative risk associated with genotypes was estimated by the odds ratio formula. P-values of less than 0.05 were considered significant.
3. Results
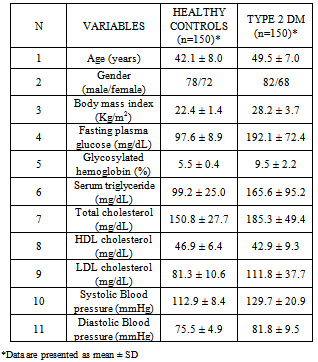
- The demographic and laboratory characteristics of the study subjects were shown in (Table 1). The mean age was 42.1 ± 8.0yrs in healthy controls and 49.5 ± 7.0yrs in T2DM (p < 0.001), the gender split of the patients was 82 (55%) females and 68 (45%) males, and for the control group, it was 78 (52%) females and 72 (48%) males. In addition there was a statistical significant difference noted in age, HbA1c (5.5 ± 0.4 vs. 9.5 ± 2.2; p <0.001), fasting blood sugar (97.6 ± 8.9 vs. 192.1 ± 72.4; p < 0.001), systolic blood pressure (112.9 ± 8.4 vs. 129.7 ± 20.9; p < 0.001), diastolic blood pressure (75.5 ± 4.9 vs. 81.8 ± 9.5; p <0.001), triglyceride levels (99.2 ± 25.0 vs. 165.6 ± 95.2; p < 0.001), total cholesterol (150.8 ± 27.7 vs. 185.3 ± 49.4; p < 0.001), and in the LDL cholesterol (81.3 ± 10.6 vs.111.8 ± 37.7; p <0.001) between the healthy controls and T2DM.The amplified PCR product of SDF-1β gene covers the +801 region and had a molecular size of 302 bp. After restriction digestion, the 302 bp product yielded two bands at 100 and 202 bp for the wild gene. For heterozygous mutants (A/G), the bands were at 302, 202, and 100 bp and for homozygous mutant (A/A) there was only a single band at 302 bp (Figure 1). Evaluation of the G801A polymorphisms in SDF-1β by MspI restriction digestion revealed that the prevalence of the G/G genotype was 25 (17.0%) in patients and 6 (4.0%) in controls. The frequency of G/A genotype was 109 (73.0%) and 131 (87.0%) in patients and controls, respectively and the frequency of the A/A genotype in patients was 16 (10.0%), and in controls, it was 13 (9.0%) (Table 2). The frequency of G allele was 159 (53.0%) and 143 (48.0%) in patients and controls, respectively. In the cases of A allele, 141 (47.0%) were observed in patients, and the frequency of this allele was 157 (52.0%) in controls (Table 2).The odds ratio of SDF-1β genotypes and alleles were calculated in T2DM compared with control subjects. The frequency of SDF-1β G/G homozygous wild in patients with T2DM showed a significantly high relative risk, when compared with healthy controls (relative risk is 4.800) which is found to be associated with this genotype (P<0.001) (Table 2). The frequency of SDF-1β G/A heterozygote observed between T2DM patients and healthy controls which showed a significant negative relative risk of 0.386, (P =0.002). Conversely the frequency of SDF-1β A/A homozygous mutant in patients with T2DM showed a relative risk of 1.258 when compared to healthy controls, which was not significantly associated with this genotype (P =0.696). The overall allelic frequency of SDF-1β G/G allele in either homozygous or heterozygous condition showed the relative risk to be 1.238 in T2DM patients compared to healthy controls with a p-value of 0.221.
|
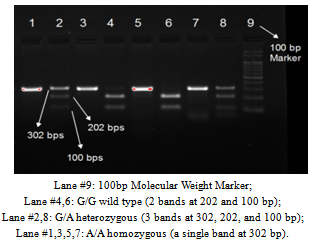 | Figure 1. PCR-RFLP results of SDF-1β Gene on 2.0% agarose gel |
4. Discussion
- Cytokines are a group of pharmacologically active low molecular weight proteins that possess autocrine and paracrine effects and are known products and effectors of the immune cells. When they are produced locally in the inflamed plaques, as frequently seen in patients with uncontrolled diabetes they exert prothrombotic effects on endothelial cells, may increase capillary permeability and cause oxidative stress and endothelial dysfunction, further aggravating the atherosclerotic process. Several situations including infection, hormonal conditions, and cytokine gene polymorphisms have been described to regulate expression and secretion of cytokines[22]. But it seems that the immune system plays an important role in etiology and pathogenesis of T2DM and its associated complications[22]. Therefore, the aim of the current study was to investigate the potential correlation of the SDF-1β G801A polymorphism with 150 south Indian T2DM patients. The results show that SDF-1β gene polymorphism is associated in patients with T2DM when compared to healthy control. Here, we observed that the AA (Mutant) genotypes of T2DM patients didn’t show any statistical significant difference when compared with healthy individuals in the SDF-1β gene. “A” allele is being considered as the mutant form of SDF-1β gene in the 801 region, whereas in the present study the frequency of this allele is found to be increased in controls (52%) than in patients (47%). This result is found to be in consistent to a recently published study from Iranian T2DM population by Derakshan et al., who had shown the frequency of this mutant “A” allele to be increased in healthy controls (16.3%) when compared to T2DM patients (15.3%) [23]. In the present study, the “GG” (Wild) genotype of SDF-1β showed a statistical significant difference than those of healthy controls. “G” allele is being considered as the wild form of SDF-1β gene in the 801 region, but our results show that the frequency of this allele is found to be increased in T2DM patients (17%) than in the control group (4%). This result is again found to be in consistent with an earlier report by Derakhshan et al., from Iranian population, where the author had shown the frequency of wild “G” allele to be increased in T2DM patients (34.8%) when compared to healthy controls (33.8%), but their significance was lost [23]. Unpublished experience from our study, show that the G/A (Carrier) genotype to be increased in healthy controls when compared with T2DM. Results of our study, showed a statistical significant difference in this genotype. This suggests that most of the healthy controls from our study population are naturally had this genotype to be polymorphic naturally. The overall apparent disparity between the Derakhshan et al., study and our study could be that both the ethnic populations are different and there is a difference among the sample volume analyzed [23].Taking together, the results of our current work indicate that the SDF-1β “G” to be the risk allele and SDF-1β “A” allele is rather a protective allele for T2DM atleast in our population. The result of our study is found to be in consistent with a previous report studied from the northern part of our Indian population by Chaudhary et al., [24]. There the author had investigated the association of SDF-1 in high risk sero-negative and HIV-1 positive patients. They had observed that the frequency of wild “G” allele to be increased in HIV-1 positive patients (91.5%) when compared to healthy individuals (79.5), showing “G” allele to be the risk for HIV-1 patients and on the other hand the frequency of mutant “A” allele to be decreased in HIV-1 positive patients (8.5%) when compared to healthy individuals (20.5), showing “A” allele to be the protective for HIV-1 patients. Another study from Greeks population by Vairaktaris et al., had investigated the association of SDF-1 with the advanced stages of oral cancer [25]. There, the author had shown the prevalence of mutant “A” allele frequency to be 25.3% in controls, and 23.2% and 12.5% in patients with cancer stages I &II and patients with cancer stages III & IV respectively. There was a statistical significant difference found between the control group and patients with cancer stages III & IV (P=0.005).
5. Conclusions
- The “GG” genotype of SDF-1β gene is confined to be the risk allele and is associated with T2DM patient’s atleast in our South Indian population. The present study did not examine levels of cytokines or SDF-1β specifically. To our knowledge, this is the first such study on SDF-1β 3’A G801A polymorphism showing the protective role of this polymorphism in T2DM patients. The strength of our present study is all the patients and controls are of the same ethnic origin. Furthermore, all of the subjects were examined in a standardized manner with well-defined diagnostic criteria. Moreover very limited reports are available on SDF-1β gene polymorphism in T2DM patients; hence studies in different populations with larger sample sizes are required to replicate these findings.
ACKNOWLEDGEMENTS
- This study was supported by a research grant from Prof.M.Viswanathan Diabetes Research Centre. We would like to acknowledge the patients with diabetes and healthy controls who contributed to this research. We again take this opportunity to thank all consultants of M.V.Hospital for Diabetes, and a special thanks to Dr.Sivakamasundari Pichu, Dr.Satyavani Kumpatla, Mrs.Usha Ganguly, Mrs.Deepa Vimalanathan, Dr.Narayana Rao, Dr.Killivallavan, Mr.Vijay Bharat and Mrs.Saradha for their immense support and assistance in submitting the manuscript.
References
| [1] | Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006 May;119(5 Suppl 1): S10-6. |
| [2] | Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits (review). Annu Rev Genomics Hum Genet. 2003 Jun; 4:257-91. |
| [3] | Malecki MT, Klupa T. Type 2 diabetes mellitus: from genes to disease. Pharmacol Rep. 2005 Aug; 57 Suppl:20-32. |
| [4] | Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001 Aug;60(3):349-56. |
| [5] | Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003 Aug; 3(4):293-8. |
| [6] | Giulietti A, Van Etten E, Overbergh L, et al. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-dihydroxyvitamin D(3) works as anti-inflammatory. Diabetes Res Clin Pract. 2007 Jul;77(1):47-57. |
| [7] | Hassanshahi G, Patel SS, Jafarzadeh AA, et al. Expression of CXC chemokine IP-10/Mob-1 by primary hepatocytes following heat shock. Saudi Med J. 2007 Apr;28(4):514-8. |
| [8] | Hassanshahi G, Jafarzadeh A, James DA. Expression of stromal derived factor alpha (SDF-1 alpha) by primary hepatocytes following isolation and heat shock stimulation. Iran J Allergy Asthma Immunol. 2008 Jun;7(2):61-8. |
| [9] | Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006 Mar 1;107(5):1761-7. |
| [10] | Lazarini F, Tham TN, Casanova P, et al. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003 Apr 15;42(2):139-48. |
| [11] | Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4 tissue-committed stem cells. Biol Cell. 2005 Feb;97(2):133-46.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF-1) gene. Genomics. 1995 Aug 10;28(3): 495-500. |
| [12] | Tashiro K, Tada H, Heilker R, et al. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993 Jul 30;261(5121):600-3. |
| [13] | Winkler C, Modi W, Smith MW, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. Science. 1998 Jan 16;279(5349):389-93. |
| [14] | Kawasaki E, Abiru NA, Kobayashi M, et al. Stromal cell-derived factor-1 chemokine gene variant in patients with type 1 diabetes and autoimmune thyroid disease. Ann N Y Acad Sci. 2004 Dec;1037:79-83. |
| [15] | Dean M, Carington M, O’Brien SJ. Balanced polymorphism selected by genetic versus infectious human disease. Annu Rev Genomics Hum Genet. 2002;3:263-92. |
| [16] | Barcellos LF, Schito AM, Rimmler JB, et al. CC-chemokine receptor 5 polymorphism and age of onset in familial multiple sclerosis. Immunogenetics. 2000 Apr;51(4-5):281-8. |
| [17] | De Oliveira Cavassin GG, De Lucca FL, Delgado Andre N, et al. Molecular investigation of the stromal cell-derived factor-1 chemokine in lymphoid leukemia and lymphoma patients from Brazil. Blood Cells Mol Dis. 2004 Jul-Aug;33(1):90-3. |
| [18] | Razmkhah M, Doroudchi M, Ghayumi SM, et al. Stromal cell-derived factor-1 (SDF-1) gene and susceptibility of Iranian patients with lung cancer. Lung Cancer. 2005 Sep;49(3):311-5. |
| [19] | Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual, Cold Spring Harbor, New York: Cold Spring Harbor Laboratory. 1982 42:149-51. |
| [20] | Balotta C, Bagnarelli P, Corvasce S, et al. Identification of two distinct subsets of long term non progressors with divergent viral activity by stromal cell derived factor 1chemokine gene polymorphism analysis. J Infect Dis. 1999 Aug;180(2):285-9. |
| [21] | Arababadi MK, Nosratabadi R, Hassanshahi G, et al. Nephropathic complication of type-2 diabetes is following pattern of autoimmune diseases? Diabetes Res Clin Pract. 2010 Jan;87(1):33-7. |
| [22] | Derakhshan R, Arababadi MK, Ahmadi Z, et al. Increased Circulating Levels of SDF-1 (CXCL12) in Type 2 Diabetic Patients Are Correlated to Disease State but Are Unrelated to Polymorphism of the SDF-1β Gene in the Iranian Population. Inflammation. 2012 Jun;35(3):900-4. |
| [23] | Chaudhary O, Rajsekara K, Ahmeda I, et al. Polymorphic variants in DC-SIGN, DC-SIGNR and SDF-1 in high risk seronegative and HIV-1 patients in Northern Asian Indians. J Clin Virol. 2008 Oct;43(2):196-201. |
| [24] | Vairaktaris E, Vylliotis A, Spyridonodou S, et al. A DNA Polymorphism of Stromal-derived Factor-1is Associated with Advanced Stages of Oral Cancer. Anticancer Res. 2008 Jan-Feb;28(1A):271-5. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML