-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Genetic Engineering
p-ISSN: 2167-7239 e-ISSN: 2167-7220
2012; 2(4): 33-37
doi: 10.5923/j.ijge.20120204.01
Association between TGF- β1-509 Gene Polymorphism with Aggressive Periodontitis
Hamid Reza Arab 1, Jalil Tavakkol Afshari 2, Mehrdad Radvar 1, Amir Moeen taghavi 1, Naser Sargolzaee 1, Majid Reza Mokhtari 1, Fateme Farazi 3
1Dept of Periodontology,School of Dentistry,Mashhad University of Medical Sciences,Mashhad,91889,Iran
2Dept of Immonogenetics,School of Medicine,Mashhad University of Medical Sciences,Mashhad,91747,Iran
3Dept of Oral Medicine,School of Dentistry,Bojnourd University of Medical Sciences,Bojnourd,49815,Iran
Correspondence to: Majid Reza Mokhtari , Dept of Periodontology,School of Dentistry,Mashhad University of Medical Sciences,Mashhad,91889,Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
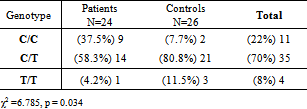
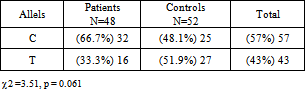
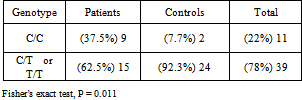
Aggressive periodontitis is a multifactorial disease characterized by progressive and relatively rapid destruction of tooth supporting tissues in otherwise healthy adolescents and young adults. It results from bacterial plaque and is influenced by environmental and genetic factors. Cytokines and growth factors play important roles in the pathogenesis of periodontitis. The aim of the present study was to investigate the relationship between the growth factor TGF-β1 (-509, C/T) gene polymorphism and generalized aggressive periodontitis (GAP). This study included 24 subjects with GAP and 26 periodontally healthy controls. Extracted DNA from peripheral blood was evaluated by PCR- RFLP method. Data were analyzed using the chi-square and Fisher’s exact tests.There was significant association between C/C genotype and GAP disease (p=0.011). The frequency of C/C genotype in patients and control subjects were 37.5% and 7.7%, respectively. The allele C was seen at 66.7% in the group with periodontitis and 48.1% in the healthy group (p=0.061). These results demonstrated that subjects with C/C genotype of the TGF-β1 polymorphism at -509 position might be more prone to the risk of developing aggressive periodontitis as compared to other genotypes.
Keywords: Periodontitis, Gene Polymorphism, TGF-Β, PCR-RFLP
Cite this paper: Hamid Reza Arab , Jalil Tavakkol Afshari , Mehrdad Radvar , Amir Moeen taghavi , Naser Sargolzaee , Majid Reza Mokhtari , Fateme Farazi , "Association between TGF- β1-509 Gene Polymorphism with Aggressive Periodontitis", International Journal of Genetic Engineering, Vol. 2 No. 4, 2012, pp. 33-37. doi: 10.5923/j.ijge.20120204.01.
Article Outline
1. Introduction
- Generalized aggressive periodontitis is a multifactorial disease characterized by progressive and relatively rapid destruction of tooth supporting tissues in otherwise healthy adolescents and young adults.Although bacterial plaque has been implicated as the primary etiologic agent in most forms of periodontal disease, there are several factors including genetic predisposition, local and systemic conditions, environment-gene interactions and etc which may affect the progression and development of the disease.[1-2]Epidemiological studies suggest that different host responses to bacteria which are contributed to genetical factors can significantly affect on clinical status. It is also claimed that about half of clinical changes in chronic peridontitis is related to genetical factors.[3]The majority of the tissue destruction in periodontitis may not directly be the effect of microorganisms but mostly is host mediated.[1, 4] In fact pro-inflammatory cytokines and chemokines released by both resident and emigrant cells at the site of inflammation are considered potentially crucial variants influencing the pathogenesis of periodontistis.[5] Several cytokines such as IL-1 (α, β, RN),TGF-β TNF- and IL-10 involve in the inflammatory and immune responses in the inflamed periodontal tissues during pathogenesis of periodontitis.[6-10]Transforming growth factor beta-1 (TGF-β1) is a multifunctional cytokine that regulates cell growth, differentiation and matrix Production.[11] It stimulates the synthesis of both matrix proteins (e.g. Collagen and fibronectin) and proteinase inhibitors (e.g. TIMP and plasminogen–activator-inhibtor-1, PAI-1) and decreases the synthesis of MMPs. In fact TGF-β1 is an antagonist for IL-1[12] Human TGF-β1 gene is located on chromosome 19q13.1-13.3 Several polymorphisms in TGF-β1 gene have recently been identified including +915 (Arg/Pro), +988 (C/A), -509(C/T), -800(G/A) and two others in 10 and 25 codones of first exon.[13] Genetic polymorphisms in the TGF-β1 gene were shown to interfere with the transcriptional activity of this gene which influence the production, secretion or activity of this growth Factor.[14-16] For example the +915 (Arg/Pro),-509(C/T) and -800(G/A) SNPs which are considered functional polymorphisms result to increased plasma concentration of TGF-B.[17] There are a lot of studies investigating the association between different biological mediators polymorphisms and periodontal disease, however data are limited about the role of TGF-β1 gene polymorphisms in the pathogenesis of periodontitis. In a previous study, de Sauza et al showed that -509(C/T) polymorphisms of TGF-β1 gene have a limited effect on gingival inflammation in the Brazilian population with chronic periodontitis[8] In another population, Holla et al couldn't find any association between -988(C/A), -800(G/A), -509(C/T), codon 10 and codon 25 polymorphisms of TGF-β1 and susceptibility to chronic periodontitis in a Czech population.[17]Gül Atilla et al reported that TGF-β1 (+915C) polymorphic allele might be associated with chronic periodontitis in the Turkish Population.[18]TGF-β1 gene polymorphisms could differ in a different population. Therefore, the aim of the present study was to evaluate the TGF-β1- 509 (C/T) gene polymorphisms in an Iranian population with generalized aggressive periodontitis and to investigate the association between TGF-β1 genotype and clinical periodontal parameters.
2. Materials and Methods
2.1. Study Population
- This case-control study was conducted at the Department of Periodontology, Mashhad Dental School by collaborating with Bu-Ali Research Institute, Mashhad University of Medical Sciences in 2009. The study protocol was approved by the ethical committee of the Mashhad University of Medical Sciences. A total of fifty unrelated, nonsmokingIranian-Khorasanian (North-East of Iran) subjects were selected. Twenty four patients (18 females, 6 males) were affected by generalized aggressive periodontitis. The diagnosis of periodontitis was based on past dental history, clinical parameters, and radiographic patterns of bone loss. Sets of intraoral radiographs were obtained using a standardized parallel technique. Patients had to be under age 30 with generalized proximal attachment loss ≥ 3milimeters at least in 3 permanent teeth other than first molars and incisors. Twenty six subjects under age 30 (16 female, 10 male) who were periodontally healthy, recruited as control group. Patients who were under inflammatory orimmunosuppressive medicine, pregnant women, drug addicted, patients who had diabetes, HIV or HTLV-1 infection or any other systemic condition that could negatively influence oral health have been excluded from the study. Written informed consent was obtained from participants.
2.2. Genotype Identification
- Blood samples, 10 milliliters from each subject, were collected by venipuncture from the arm vein, mixed with EDTA and stored in -70℃ until further processing. DNA was isolated using non-enzymatic "salting out" method by means of BioGene® whole blood kit (Mashhad, Iran).DNA samples were subjected to polymerase chain reaction (PCR) using the specific primers with following sequences: Sense; 5’- TTT TGC CAT GTG CCC AGT AG-3’ and Antisense; 5’- CAC CAG AGA AAG AGG ACC AG-3’(GenBank accession No. X05839). The PCR amplifications were performed in 20 μl volume containing 100-150 ng DNA, 500 μmol of specific primers, 0.5 unit Taq DNA polymerase, 10x PCR reaction buffer (10mM/L Tris-HCl, 50 mM/L KCl, 1.5 mM/L MgCl2) , 0.2 mM each dNTP .Amplification was performed by a thermocycler (Corbet Research, Australia, model GP-001) under the following conditions; 1 cycle at 95℃ for 2 min, 35 cycles each at 95℃ for 1 min, 62℃ for 1min and 72℃ for 1 min, an extension time of 5 min at 72℃ was used for 1 cycle.The products of PCR were visualized by electrophrosis on a 1.5% agarose gel stained with Etidium bromide. Then they were digested by restriction enzyme Eco 81I (20U/ml) (Fermentas, Germany) for 3 hours and cleaved DNA fragments were subjected to electrophoresis on 17% polyacrylamide gel and stained with silver nitrate. (Figure.1)
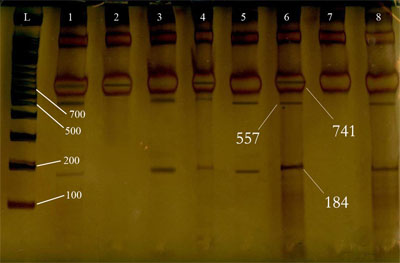 | Figure 1. PCR products after RFLP, staining with AgNo3. Uncut -homozygote (CC): 2. Cut-homozygote (TT): 3, 5 and 8. Cut-heterozygote (CT): 1, 4 And 6 |
2.3. Data Analysis
- Allelic frequencies of TGF-β1-509 were calculated from the observed number of genotypes and expressed as the percentage of the total number of alleles. Deviation of genotype distribution from Hardy-Weinberg equilibrium was assessed by chi-square test. Differences in allele frequencies and genotypes distributions between the study groups (cases and controls) were also analysed by chi-square test and Fisher's exact test. P values less than 0.05 were considered statistically significance.The data analysis was accomplished using the "STATA 7" software.
3. Results
3.1. Genotype Frequency
- Table 1 demonstrates the frequency of different genotypes with respect to their periodontal status. However, the results of the Chi-square test could not be considered valid because one third of the cells of the chi-square 2 by 3 contingency table had expected counts of lower than 5 (Table1).
|
3.2. Allele Frequency
- Table 2 shows the allele frequencies of the study groups. The frequency of allele C was greater among the GAPs, while the frequency of allele T was greater among the control subjects. However, the differences were not statistically significant. (χ2=3.51 and p = 0.061, Table2).
|
|
4. Discussion
- Transforming Growth Factor β1 (TGF-β1) is one of the cytokines involved in the complex mechanism of periodontal diseases[8] The higher concentration of TGF-β1 has been detected from periodontal tissue and gingival pocket fluid of patients with chronic periodontitis.[19-20] This cytokine has an anti-inflammatory and immunosuppressive function and because of its effect on connective tissue, remodeling and bone metabolism, it has a key role in pathogenesis of periodontal disease.[12, 21] The large number of previous related studies examining polymorphisms of the TGF-β1 gene in various diseases reflects the interest in the role of this gene in chronic inflammatory diseases.[22] Yamada et al reported significant association between the 509C/T polymorphism and bone mineral density in postmenopausal Japanese women.[23] It was suggested that 713-8delC polymorphism is associated with low bone density in Italian and Danish postmenopausal women.[24-25]It is well known that genetic variance is a major determinant of the differential risk for many human diseases including periodontitis. Genetic alteration could change the transcript level of the protein. The results can range along a continuum of functional consequences, from no observable change in protein function, a minor change in function, to a dramatic change or obliteration of function.[26] TGF-β1 has several functional polymorphisms[17] Eight polymorphisms in the TGF-β1 gene have recently been identified including +915G/C in exon 1, Thr263Ile in exon 5 and 713-8delC in intron 4.[25, 27-29]The -590 polymorphism is located in a negative regulatory region (-731 to -453) previously determined to be associated with a decreased transcription of the TGF-β1. In this study, we have evaluated the common gene polymorphism of TGF-β1 at -509 C to T .Our result showed that the frequency of C/C genotype is significantly more in GAPs compared with control group. However, only marginally significant differences were found in allele frequencies between two groups. Our results consistent with those of Holla et al. (2002) and de Souza et al. (2003) which showed that the C/T genotype was the most prevalent genotype among the normal population[17].In addition, our study is consistent with those of Holla et al. (2002) in finding a more prevalent T/T genotype among the periodontally healthy subjects as compared to the periodontitis patients. Although these findings were not supported by de Souza et al (2003) who found the frequency of TT genotype higher in the severe periodontitis than in the control or moderate groups[8]. Different type of periodontitis can be an explanation for the difference between our result and de Souza's; in our study population was patients with generalized aggressive periodontitis whereas Souza's target group was chronic periodontitis patients. Another possible reason can be the racial differences, (Iranian vs Brazilian)An epidemiological survey demonstrated that the prevalence and gender ratio vary geographically and/or racially in AgP[30]In this study it has been observed that significantly greater proportion of subjects with C/C genotype suffered from GAP compared to other combined genotypes (T/T and C/T). This might be due to the fact that C/C genotype subjects produce greater amounts of TGF- β1 prohibiting the action of proinflammatory cytokines such as IL- β1. Steinsvoll et al (1999) showed that subjects with periodontitis have an upregulated TGF-β. Most of the studies investigating the polymorphisms of TGF-β1 are focused on chronic diseases. Some of them have found significant association between TGF-β1polymorphism and diseases and some did not support such relationships. [15, 31] In spite of these studies, establishing whether an association exists between a gene polymorphisms and a multifactorial disease is a difficult task. It is given that there are around 10 distinct single nucleotide polymorphisms for every 10 kb nucleotide in human genome, of which most of them are considered silent[32] Therefore, it seems more logical that when evaluating the association of a multifactorial disease such as periodontitis with gene polymorphism, a few gene polymorphism should be evaluated simultaneously.
5. Conclusions
- Our results indicated that the C/C genotype of the -509 polymorphism of the TGF-β1 gene increases the risk of aggressive periodontitis. It might be an essential factor in the progression of the bone loss and attachment loss in GPA patient, at least in the Iranian population. However, considering the relatively small number of the studied subjects, more extensive studies in larger groups of patients should be undertaken in order to analyse the putative association of the TGF-β1 polymorphism in the pathogenesis of periodontitis.
ACKNOWLEDGEMENTS
- This study was supported by the Vice Chancellor for Research, Mashhad University of Medical Sciences (MUMS), Mashhad, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML