-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2025; 15(1): 14-23
doi:10.5923/j.ijee.20251501.02
Received: May 30, 2025; Accepted: Jun. 27, 2025; Published: Jul. 25, 2025

Energy Study of Gases Obtained by Gasification of Agroforestry Residues
Abdoulaye Hachi Alio1, Harouna Gado Ibrahim2, Doulla Seydou Nouhou1, Boukar Makinta1
1Department of Physics, Abdou Moumouni University, Niamey, Niger
2Department of Physics, Dan Dicko Dankoulodo University, Maradi, Niger
Correspondence to: Boukar Makinta, Department of Physics, Abdou Moumouni University, Niamey, Niger.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

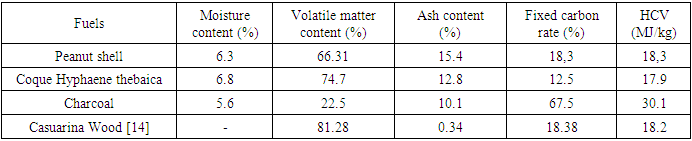
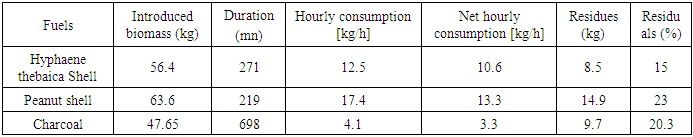
Fossil fuels still dominate the global energy mix, while the use of these sources has harmful consequences for our environment. This requires a look at other, cleaner sources for the security of our planet. This is how biomass is perceived in Niger as one of the solutions for an energy transition with a significant agroforestry potential available for bioenergy. Hence the main objective of the present study, which focuses on the gasification of agroforestry residues. The gasifier used is of the co-current fixed bed type, and the three fuels used are peanut shell, Hyphaene thebaica shell and charcoal. The fuels were first characterized according to recommended standards and some correlations to estimate the higher calorific value. During the gasification, reduction temperatures were recorded, with averages ranging from 713.3°C for groundnut shell, 850°C for Hyphaene thebaica shell and 861°C for charcoal. The gases produced are well purified by the use of an exchanger. This is because the latter cools the gases to the point of optimal removal of impurities. Estimated gasification yields range from 60.7% for groundnut hull and 62.1% for Hyphaene thebaica hull and 37% for charcoal. The gasifier power varies respectively from 12.7 kWth for charcoal gasification, 38.6 kWth for the Hyphaene thebaica hull and 53.8 kWth for the peanut shell. These differences in results from one fuel to another are related to the different operating parameters and the characteristics of the fuels. The gas produced can be used directly as domestic gas to turn the shaft of a turbine.
Keywords: Gasification, Residues, Agroforestry, Co-current fixed bed, Peanut shell, Hull of Hyphaene thebaica, Charcoal
Cite this paper: Abdoulaye Hachi Alio, Harouna Gado Ibrahim, Doulla Seydou Nouhou, Boukar Makinta, Energy Study of Gases Obtained by Gasification of Agroforestry Residues, International Journal of Energy Engineering, Vol. 15 No. 1, 2025, pp. 14-23. doi: 10.5923/j.ijee.20251501.02.
Article Outline
1. Introduction
- Global energy demand is currently growing by an average of 2% per year [1] and according to the IEA (International Energy Agency) report of 2022, this demand is based on a dominance of fossil fuels up to 80% [2]. While the use of these fossil sources contributes to the degradation of our planet and prevents developing countries from evolving because of their dependence on these sources. In addition, these energies are not renewable and their prices are constantly rising [3]. In addition, they are largely a source of greenhouse gas emissions that are at the origin of global warming, which massively disrupts ecosystems and threatens human societies [4].In the case of Niger, final energy consumption estimated at 0.13 toe (ton oil equivalent) per capita in 2019 and is one of the lowest in the world (lower than the African average of 0.65 toe and the world average of 1.88 toe) [5]. This weakness could be explained mainly by a limited supply, very high population growth, a poorly developed industrial sector and very low purchasing power, and on the other hand by limited access of Nigerien households to modern energies because traditional biomass remains the first source of energy used to the tune of 80.65% [5].Thus, in the face of these energy challenges, a synergy of alternatives is more than necessary, including the use of renewable energy sources, the improvement of energy efficiency, the reduction of greenhouse gas emissions and the promotion of the use of cleaner fuels; This is how biomass is perceived as one of the key solutions for the energy transition in Niger. Indeed, according to the FAO (Food and Agriculture Organization) in 2010, about 1204000 ha of Niger is forested, i.e. 1% of the national territory [6]. In addition, in a study carried out in 2011, it was reported that Niger recorded 2672 million tons of agricultural residues and that the use of 30% of these residues could make it possible to obtain a potential of 100MW [7]. Another study in 2020 reported that in Niger, on average over 10 years, the production of certain agricultural products dominated by millet followed by sorghum and groundnuts generated an estimated gross quantity of residues of 11,835,400 tons, of which 2,255,471 tons were available for bioenergy, i.e. 19% [8]. However, the production of residues of forest species such as Balanites aegyptiaca and Hyphaene thebaica amounts to 528580 tons and 17782 tons respectively, of which 88400 tons of these residues can be mobilized in bioenergy, i.e. 12.5% of the raw residues [8]. In view of the above, biomass (particularly agroforestry residues) can be an alternative source to support the use of LPG (Liquefied Petroleum Gas) gas as cooking gas in Niger.Based on the energy potential in agricultural and forestry residues that Niger has and on the other hand on several studies carried out on the feasibility of valorizing the latter into biogas, the need arises to deal with this theme which would rule on an energy study of the gases obtained by gasification of agroforestry residues. This study aims first at the possibility of developing a gasification system for agroforestry residues and secondly at the evaluation of the energy quality of agroforestry residues.
2. Material and Methods
2.1. Material
2.1.1. Gasifier
- The gasifier of the study is a co-current fixed-bed gasifier installed on the site of the Dan Dicko Dankoulodo University of Maradi. This system is made up of four (04) main parts (Figures 1 and 2):
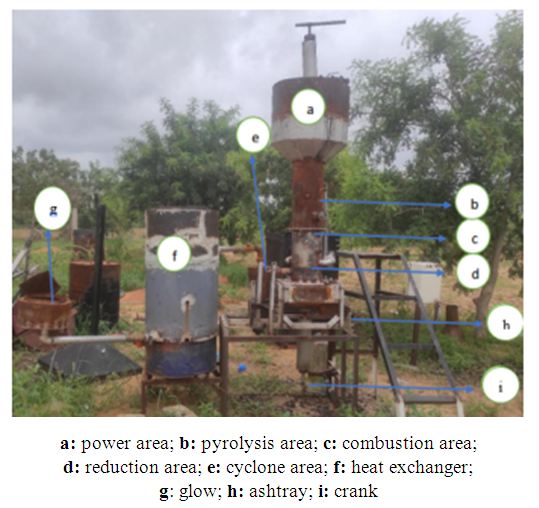 | Figure 1. Study design |
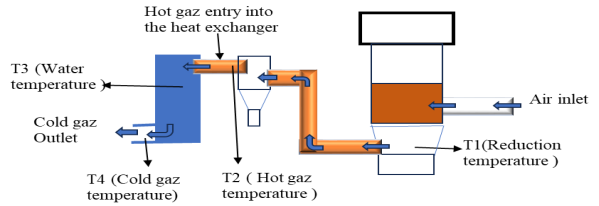 | Figure 2. Schematic of the device |
2.1.2. Material for Immediate Analysis
- The immediate analysis was carried out at the physics laboratory level of the Faculty of Science and Technology of the Dan Dicko Dankoulodo University of Maradi. It consists of determining the moisture content, ash content, volatile matter content and fixed carbon level in the biomass. The main devices used are, among others: Ø A 220g ± 0.1mg capacity scale was used to measure the masses of the samples analyzed; Ø An oven to dry the biomass; Ø A RESISTANCE FURNACA brand furnace which was used to determine the level of ash and volatile matter;
2.1.3. Fuels
- The different fuels used in this study are groundnut shell, Hyphaene thebaica shell and charcoal (Figure 3). All these biomasses are dry and they were found at dealers in the city of Maradi.
 | Figure 3. (A) Hyphaene thebaica shell, (B) Peanut shell, (C) Charcoal |
2.2. Methods
2.2.1. Gasification Methodology
- The methodology suitable for gasification tests is structured in different steps which are detailed below: The first gasification step is the loading of biomass into the gasifier. The biomass is first weighed and then introduced into the device through the flange at the top (Figure 4).
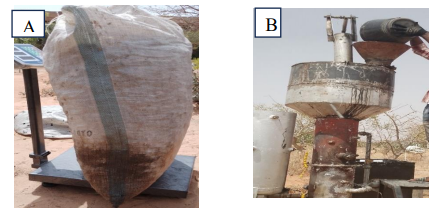 | Figure 4. (A) biomass weighing, (B) biomass loading |
 | Figure 5. Ignition of the gasifier |
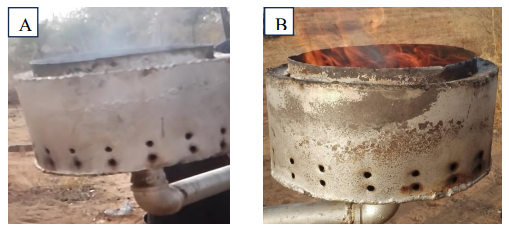 | Figure 6. (A) non-flammable gas; (B) Flammable gas |
 | Figure 7. (A) reactor feed, (B) ash removal |
 | Figure 8. (A) residues disposal, (B) Residue weighing |
2.2.2. Fuel Characterization Methodology
- Characterization consists of making an immediate analysis of the fuels by determining the contents of moisture, volatile matter, ash and fixed carbon and estimating the calorific value of the fuels based on the results of the immediate analysis.
2.2.2.1. The Moisture Content
- The moisture content of biomass is determined using EN 14774-3:2009 [9]. The procedure is that of first weighing the mass m0 (of the vacuum crucible), then a mass of sample is transferred to the crucible, then the mass m1 (of the crucible + the sample before drying) is weighed. The crucible that contains the sample is then placed in the oven and then set the temperature of the oven to 105°C until a constant mass is obtained. At the exit of the oven, the crucible is placed in the desiccant for 15 minutes and then the new mass m2 (crucible + the dehydrated sample) is weighed. Thus, the moisture content (in percentage) is calculated by the following relationship:
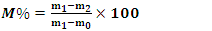 | (1) |
2.2.2.2. The Ash Content
- The ash content is determined using EN 14775:2009 [10]. The test portions must be at least 1 g. The mass of the empty crucible and the mass of the crucible + sample are weighed respectively m0 and m1. Then the mass m1 is placed in the furnace at room temperature, then this temperature is set to 250°C. This temperature is maintained for 60 minutes to allow volatile substances to leave the sample prior to inflammation. Then it is still raised to (550 ±10) °C and maintained for 2 hours. After heating, the crucible is allowed to cool to room temperature and then transferred to the desiccant. The new mass m2 (of the crucible + the ashes) is weighed. Thus, the rate of ash on a dry basis is determined by the following relationship:
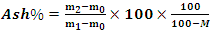 | (2) |
2.2.2.3. Volatile Matter Content
- The level of volatile matter in the biomass is determined using the muffle furnace in accordance with EN 15148:2009 [11]. The procedure consists of weighing the 2 masses m0 (crucible + lid) and m1 (crucible + lid + sample before heating) then introduce m1 into the furnace at a temperature of 900°C ± 20°C for a time of 7 minutes. When it comes out of the furnace, the new mass m2 (crucible + lid + contents after heating) are weighed. The volatile matter content of the sample, expressed as a percentage by mass, is given by the following relationship:
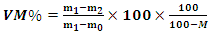 | (3) |
2.2.2.4. Fixed Carbon Content
- The quantity of carbon remaining after the removal of moisture, volatile matter and ash is deducted according to the formula:
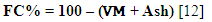 | (4) |
2.2.2.5. Calorific Value
- In the present study, the higher calorific values (HCV) of the two biomasses were estimated using equation (5):
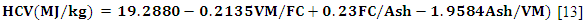 | (5) |
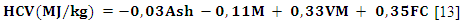 | (6) |
2.2.3. Gasifier Performance
- This part consists of estimating the energy of the gas produced and the performance of the gasifier. To do this, it is necessary to know the net hourly consumption of the device, the biomass conversion rate, the energy available in the fuel.
2.2.3.1. Duration of Gasification
- The duration of the gasification is the interval between the flammability test and the time when the flame has stopped.
2.2.3.2. Net Consumption
- The Net Consumption (Cnet) is the difference between the mass of the fuel introduced and the mass of the residues collected after gasification.
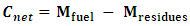 | (7) |
2.2.3.3. Hourly Consumption
- This consumption is determined by the ratio of the mass of fuel consumed to the gasification time. The expression is given by the relation (8):
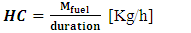 | (8) |
2.2.3.4. Net Hourly Consumption
- This quantity is determined by the ratio between the net consumption and the duration of gasification. It is denoted HCnet and given by the relation (9).
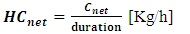 | (9) |
2.2.3.5. Fuel Energy
- It is the energy contained in the fuel introduced into the gasifier. It is given by the relation (10).
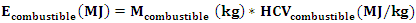 | (10) |
2.2.3.6. Fuel Conversion Rate
- It is the mass fraction of fuel converted at the end of gasification [28]. It is equal to:
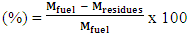 | (11) |
2.2.3.7. Mass Yield
- The mass yield is the mass fraction of the coal obtained at the end of gasification. It is obtained by the ratio between the mass of coal and the anhydrous mass of biomass introduced into the gasifier. It is determined by the relation (12).
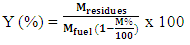 | (12) |
2.3.3.8. Net Energy
- This is the amount of fuel energy consumed during gasification. It is obtained by the relation (13).
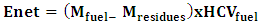 | (13) |
2.2.3.9. Gasification Efficiency
- This efficiency is the ratio of the gas energy produced to the biomass energy consumed (net energy). It is given by the relation (14).
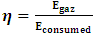 | (14) |
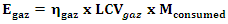 | (15) |
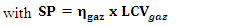 | (16) |
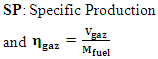 | (17) |
2.2.3.10. Thermal Power of the Gasifier
- The thermal power of the device is calculated by the product of the specific gas production by the hourly consumption of the device.
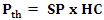 | (18) |
3. Results and Discussion
3.1. Fuel Characterization
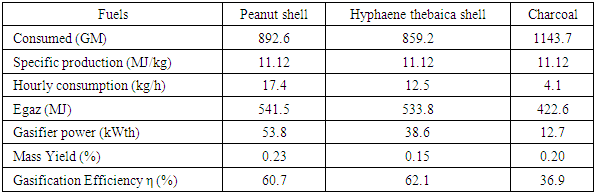
- The fuel characteristics determined in this study are the parameters from the proximate analysis and the HCV. The results of this characterization are presented in Table 1:
|
3.2. Gasification Test Results
3.2.1. Gasification Temperature Profiles (T1)
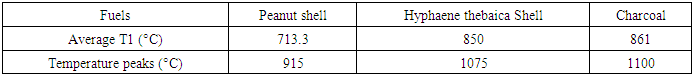
- The temperature measured during gasification is the gas reduction temperature. Its profiles are presented by the curves in Figure 9. The letters A, B and C correspond respectively to the peanut shell, the hull of Hyphaene thebaica and charcoal.
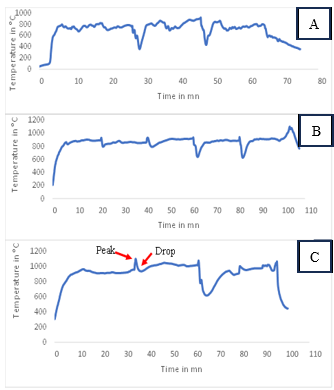 | Figure 9. Evolution of the reduction temperature T1 (A) peanut shell; (B) Hyphaene thebaica shell; (C) charcoal |
|
 | Figure 10. Ashes obtained from peanut shells |
3.2.2. Operating Conditions for Gasification Tests
- This section summarizes the information gathered during the 9 gasification tests. This information is summarized in Table 3.
|
 | Figure 11. Charcoal residues and ash disposal grate |
3.2.3. Gas Cooling
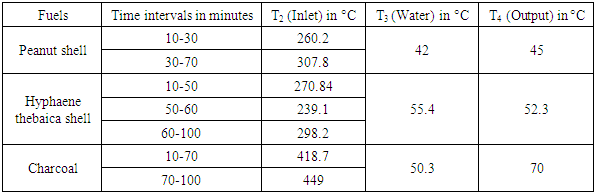
- This section will focus on the treatment of the gas at the exchanger, in particular its cooling for later use. The aim is to monitor the evolution of the gas temperatures at the inlet (T2) and outlet of the exchanger (T4) and that of the water in the exchanger (T3). These gaits are shown in Figure 12.
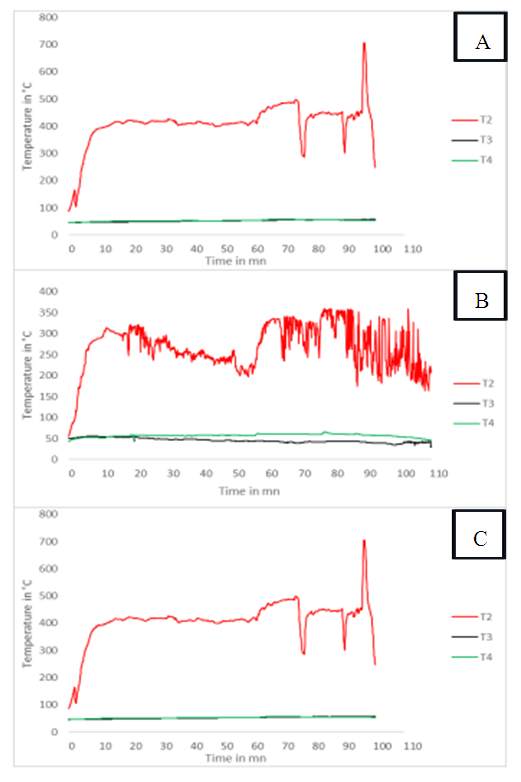 | Figure 12. Temperature evolution T2, T3, T4: (A) peanut shell; (B) Hyphaene thebaica shell; (C) charcoal |
|
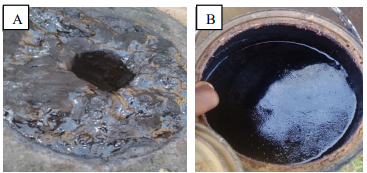 | Figure 13. (A): tar (B): condensed water |
3.3. Device Performance Estimation
- The main performance points targeted in these parts are the power of the device, the mass yields and the gasification yields.Table 5 provides an estimated overview of the performance elements of the gasifier.
|
4. Conclusions
- Our work focuses on the thermochemical route through gasification to valorize agroforestry residues. The available gasification product is a syngas typically composed of CO, H2, CH4 in small quantities and tar which is undesirable, but their proportions depend on the operating parameters and the characteristics of the biomass. For the specific case of the present study, the carbonated residues are peanut hull and Hyphaene thebaica husk and charcoal. Gasification was carried out using the co-current fixed-bed gasifier. The two shells studied have significant energy potentials with 18.3MJ/kg for the peanut shell, 17.9MJ/kg for the Hyphaene thebaica shell, but charcoal is more energetic with 30.9MJ/kg. Gasification temperatures Gasification tests are more important with coal and the hull of Hyphaene thebaica than with peanut hull with averages of 861°C, 850°C and 713.3°C respectively. The gases produced are well purified thanks to an efficient response of the exchanger. This is because the latter cools the gases to the point of optimal removal of impurities. The gasifier has gasification efficiencies estimated at 62.1% for the gasification of the Hyphaene thebaica hull and 60.7% for the peanut shell and 37% for the coal, respectively. So, the gasifier is more efficient for the gasification of the hull of Hyphaene thebaica and the hull of peanut compared to charcoal which has a low yield. These variations are related to the reactions present at the level of gas reduction. The gasifier power varies respectively from 12.7 kWth for charcoal gasification, 38.6 kWth for the Hyphaene thebaica hull and 53.8 kWth for the peanut shell. The variation in power is due to the hourly consumption of each fuel.The operation of the device and the characteristics of the fuels affect the residue levels after gasification. The study also showed that groundnut hull has a higher residue rate (23%) compared to 15% for Hyphaene thebaica hull, 20.3% for charcoal.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML