-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2023; 13(1): 13-23
doi:10.5923/j.ijee.20231301.02
Received: Mar. 5, 2023; Accepted: Mar. 22, 2023; Published: Apr. 15, 2023

Formulation and Modification of Multi-Walled Carbon Nanotubes for Post-Combustion CO2 Capture
Ateke Harriet Idaereesoari, Aimikhe Victor Joseph
Department of Petroleum & Gas Engineering, University of Port Harcourt, Nigeria
Correspondence to: Aimikhe Victor Joseph, Department of Petroleum & Gas Engineering, University of Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The emission of CO2 from fossil fuels causes environmental degradation; hence, this noxious gas must be captured and put away for environmental sustainability. Adsorption technology shows promising characteristics for CO2 capture and storage. Multi-walled carbon nanotubes have been explored for CO2 capture. However, studies on their modification with deep eutectic solvents, a class of ionic liquids with the propensity for enhancing CO2 capture, are scarce in the open literature. Thus, this study formulated multi-walled carbon nanotubes (MWCNT) from acetylene as a carbon source and modified it using deep eutectic solvent (DES) developed from choline chloride + glycerol. For comparison, polyethylene glycol (PEG), a similar alcohol-based solvent, was also used to modify the MWCNT. The CO2 adsorption capacity of the adsorbents was evaluated at temperatures of 26, 40, and 70°C and pressures of 5, 10, and 15 psi using an experimental adsorption setup designed and fabricated for this study. Results showed that unmodified MWCNT, MWCNT+PEG, and MWCNT+DES had CO2 adsorption capacities of 0.174 mmol/g, 0.220 mmol/g, and 0.226 mmol/g at 26°C and 15 psi, respectively. This trend showed that DES had a better affinity for CO2 than the unmodified MWCNT. Also, the adsorption capacities of MWCNT-DES and MWCNT+PEG were relatively the same at 26°C and 15 psi. The adsorption isotherm of the MWCNT-DES followed the typical physisorption Langmuir model. Soil test results showed that disposing of the CO2-rich adsorbents to the environment improves soil nutrients. The low adsorption capacity of the MWCNT-DES adsorbent may be due to the fact that acetylene does not produce a suitable MWCNT with an appropriate internal structure for the adsorption of CO2 under the process conditions studied. Therefore, MWCNT made from acetylene and modified with DES and PEG are unsuitable for CO2 adsorption.
Keywords: Carbon capture, Post-combustion, Nanoparticles, CO2 adsorption
Cite this paper: Ateke Harriet Idaereesoari, Aimikhe Victor Joseph, Formulation and Modification of Multi-Walled Carbon Nanotubes for Post-Combustion CO2 Capture, International Journal of Energy Engineering, Vol. 13 No. 1, 2023, pp. 13-23. doi: 10.5923/j.ijee.20231301.02.
Article Outline
1. Introduction
- The continuous emission of carbon (IV) oxide (CO2) into the environment through the exploitation of fossil fuels for energy generation and production in various process systems constitutes environmental challenges to the sustainability of our ecosystem [1]. Also, the increase in global carbon footprint poses a challenge to the actualization of the Paris Treaty of 2015 and the target of limiting global temperature rise to 1.5°C above the pre-industrial era. Therefore, this creates the need to seek innovative solutions that would forestall carbon emissions and safeguard the environment. Amid the diverse decarbonization technologies for carbon emission control and environmental change alleviation, carbon capture and storage (CCS) has become a highly viable option for decarbonizing carbon-intensive processes. CCS is a weighty module of most climate models as a contrivance to alleviate climate change in the coming decades. Pieces of literature argue that without CCS, meeting climate change goals would become an uphill task [2]. CCS is a decarbonization technology that captures carbon from process systems, e.g., coal power plants, natural gas processing, hydrogen production plants, steel, and concrete manufacturing plants, and stores it underground, safe from the environment [3]. It is based on three technologies, including pre-combustion, oxy-combustion, and post-combustion technology, as shown in Figure 1. Among these technologies, the post-combustion carbon process is the most developed and widespread, process efficient, and easily retrofitted technology for CO2 capture [4]; hence, it would be the focus technology in this study.
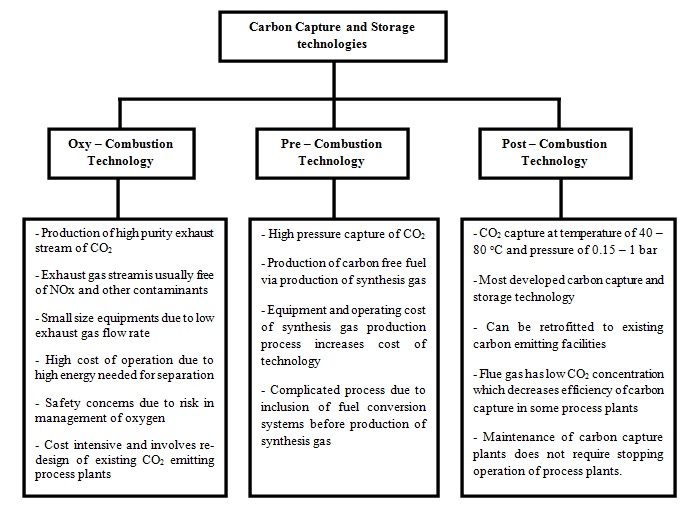 | Figure 1. Different types of CCS Technologies |
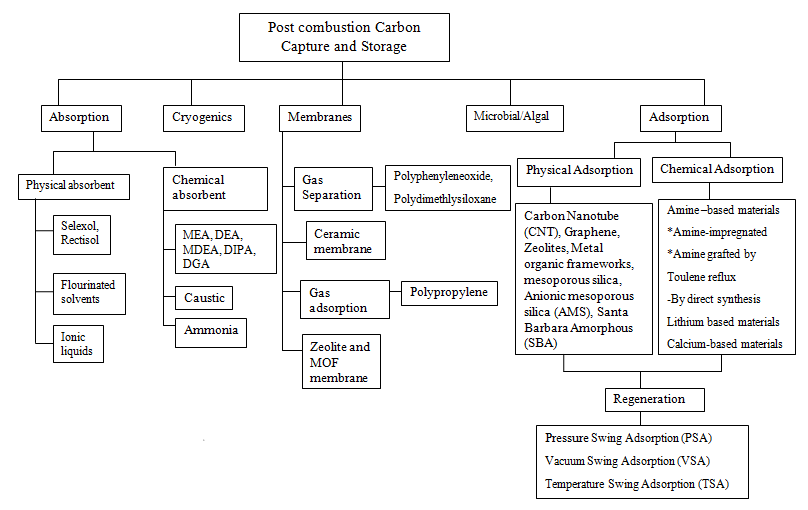 | Figure 2. Different types of post-combustion carbon capture and storage mechanisms reproduced from Aimikhe and Eyankware [1] |
1.1. Adsorbents for CCS
- Adsorption technology is a molecular-driven process that involves intermolecular interactions between adsorbate (CO2) and adsorbent (capture media). It is divided into physisorption (driven Van der Waals force) and chemisorption (stronger intermolecular forces). Physical adsorption is a reversible process mechanism driven by the Van der Waals force involving weak intermolecular interaction in the 10– 20 KJ/mol range. On the other hand, chemical adsorption is an irreversible mechanism driven by stronger chemically induced adsorption forces within a binding energy range of 10 – 100 KJ/mol. Most of the time, adsorption technology consumes less energy because of its low heat of adsorption. Hence, this capture mechanism tends to lessen the drawbacks of popular amine scrubbing solvents. This comparative advantage prompted intensive research into using adsorbents for carbon capture at post-combustion conditions. Various kinds of adsorbents like metal-organic frameworks [8], zeolites, [9] silica [10] activated carbon [11], and carbon nanoparticles have been reported as suitable adsorbents for CO2 scrubbing. The numerous application of adsorption technology in different process systems has prompted researchers to investigate advanced and novel adsorbents with improved functionalities. Materials with outstanding high surface areas (~10,000 m2/g), high porosity, selectivity, and good thermal, mechanical, and chemical stability [12]. These novel adsorbents have often outperformed traditional adsorbents. Examples include metal-organic frameworks (MOFs) [13], Li4S4O4 [14] peptide nanotubes [15], carbon nanosheet-based networks (GPC) [16], carbon nanotubes [17], etc. Among these different adsorbents, carbon nanoparticles produced from precursors like carbon black, activated carbon, graphite, biochar, charcoal, polymers, etc., have been demonstrated to be stable thermally and mechanically [18], [19]. In addition, the possibility of modification/functionalization of carbon nanoparticles and its consequent ability to increase the adsorption and selectivity capacity of pristine adsorbents further highlights the propensity of carbon nanoparticles as a suitable adsorbent for industrial and commercial CCS applications [20].
1.2. Carbon Nanoparticles
- Carbon nanoparticles are nanomaterials with particle sizes < 100 nm in two dimensions [19]. Examples of carbon nanoparticles include carbon black (CB), carbon nanotubes (CNT), and graphene derivatives. They are highly porous structures whose properties are a function of the type of carbon source from which it is derived [21]. Carbon nanoparticles possess high mechanical and thermal stability, good gas (CO2) adsorption capacity, high surface area, and permanent structure [18]. Among these different types of carbon nanoparticles, CNT [especially multi-walled carbon nanotube (MWCNT)] is one of the most well-known because of its favorable properties like resistance to temperature changes, strength and thermal stability, hydrophobicity, and availability of precursors. Hence, it is the adsorbent of focus in this study.CNT exhibits exceptional properties making them highly suitable for process applications. The mechanical strength of CNT is viewed as one of the most established because of the development of covalent sp2 bonds between individual carbon atoms, collectively forming one of the highest elastic Young Modulus and tensile strength [22]. Furthermore, experiments have shown that the outer shell graphene layer of MWCNT withstands any stress imposed on the structure when it is dispersed in a matrix [23]. Also, MWCNT can withstand temperatures of 750°C and 2800°C in a vacuum and open space, respectively, highlighting its stability. In addition, MWCNT has a surface design that can be changed to improve its affinity for gases. This further increases the appropriateness of MWCNT for adsorption applications. Incorporation of CO2-philic components such as N-compounds, amino-compounds, Lewis base, and amine solvents into structures of carbon nanoparticles have been reported to improve their adsorption properties [20], [24], [25]. Modifying the pristine structure of carbon nanoparticles creates active sites with a strong affinity for CO2. Also, it results in the alteration of the pore sizes such that specific molecule sizes are allowed into the structure, thereby increasing selectivity. Consequently, MWCNT can be considered an appropriate material for creating adsorbents for CO2 and other harmful gas capture. Specifically, deep eutectic solvents (DES) [26] and (PEG) [27] have been identified to have good adsorption properties. Also, glycols have been found to improve the absorption of CO2 in non-aqueous amines. Hence, this study will investigate them as modification agents for enhanced CO2 adsorption at post-combustion conditions.
1.3. Deep Eutectic Solvents (DES) and Polyethylene Glycol (PEG)
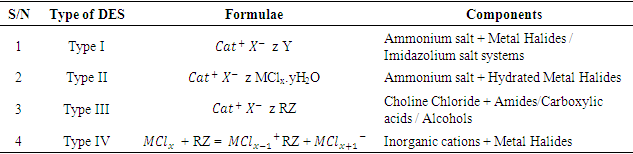
- DES is a complex compound composed of quaternary ammonium salts (hydrogen bond acceptor) and hydrogen bond donors [28]. They are also considered a sub-class of ionic liquids. DES have lower melting points than their constituting components; hence, they are called eutectics [29]. DES is usually represented using the chemical formula shown in Equation 1.
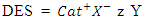 | (1) |
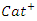 = sulphonium, ammonium, and phosphonium ion
= sulphonium, ammonium, and phosphonium ion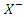 = Halide ionz = Number of molecules of Y that interact with XY = Lewis Acid\--There are four types of DES: Type I, Type II, Type III, and Type IV. Each class has distinct components (quaternary ammonium salts and HBDs) that make up the compound. These different components are highlighted in Table 1. Among these types, Type III has favorable properties such as biodegradability, simplicity in its preparation, and non-ecotoxicity. Subsequently, it is the most utilized type of DES for modifying adsorbents. Type III DES comprises choline chloride (hydrogen bond acceptor) and alcohol, carboxylic acid, and amides (hydrogen bond donor). The usefulness of DES depends on its capacity to add – NH group to the outer layer of adsorbents expanding its basicity and affinity for CO2, which is an acidic gas. Hence, the utilization of DES in modifying adsorbents for CO2 capture has been reported in the literature [26].
= Halide ionz = Number of molecules of Y that interact with XY = Lewis Acid\--There are four types of DES: Type I, Type II, Type III, and Type IV. Each class has distinct components (quaternary ammonium salts and HBDs) that make up the compound. These different components are highlighted in Table 1. Among these types, Type III has favorable properties such as biodegradability, simplicity in its preparation, and non-ecotoxicity. Subsequently, it is the most utilized type of DES for modifying adsorbents. Type III DES comprises choline chloride (hydrogen bond acceptor) and alcohol, carboxylic acid, and amides (hydrogen bond donor). The usefulness of DES depends on its capacity to add – NH group to the outer layer of adsorbents expanding its basicity and affinity for CO2, which is an acidic gas. Hence, the utilization of DES in modifying adsorbents for CO2 capture has been reported in the literature [26].
|
2. Materials and Methods
- For this study, an experimental apparatus was developed to formulate MWCNT and assess its adsorption capacity at post-combustion conditions. The formulated MWCNT was then modified with PEG and DES before conducting CO2 adsorption experiments using the apparatus described by Aimikhe and Eyankware [35].
2.1. Synthesis of MWCNT
- MWCNT was synthesized via catalytic chemical vapor deposition (CVD) using acetylene as a carbon source, argon as the carrier, and a catalyst using a fabricated setup [36]. The fabricated apparatus is shown in figure 3. The setup was initially purged using nitrogen gas, after which acetylene and argon were flown into the vaporizer and subsequently into the swirled mixer. At this point, the gas mixer was ignited with fuel from a fuel source. Consequently, vapor decomposition occurred in the Quartz reactor situated inside the tubular reactor, whose temperature was measured using a dial thermometer. The produced MWCNT was obtained from the exit trap and secondary exit traps.
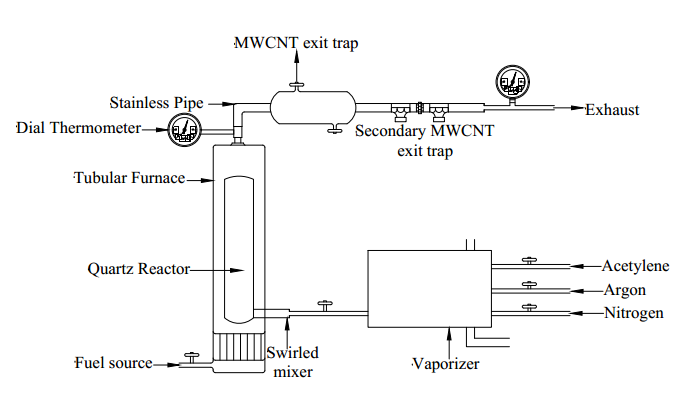 | Figure 3. Experimental setup for MWCNT Synthesis |
2.2. Modification of MWCNT
- MWCNT was modified with a solution of DES prepared from choline chloride + glycerol and polyethylene glycol (PEG). These modified samples were named MWCNT-DES and MWCNT-PEG, respectively. The DES was prepared by mixing choline chloride + glycerol in 1:2 (30g: 60g) and stirring at 80°C for 2 hours using a magnetic stirrer. DES was commingled with MWCNT in the ratio of 1:2 in a vacuum at 150°C for 4 hours, after which samples were dried at 150°C to ensure a fine form for good adsorption. To prepare MWCNT-PEG, PEG and MWCNT were commingled in the ratio of 1:2 and blended utilizing a magnetic stirrer at 100°C for 4 hours. Finally, the commingled MWCNT-PEG was dried in an oven at 150°C for 3 hours to obtain dry samples.
2.3. Characterization of MWCNT-DES and MWCNT Adsorbents
- The formulated MWCNT-DES and MWCNT adsorbents were characterized using the Infrared spectrometer (Varian 660 MidIR Dual MCT/DTGS Bundle with ATR) for the Fourier transform infrared spectroscopy (FTIR) analysis and the Hitachi SU 3500 scanning electron microscope for the SEM analysis. These analyses were performed to understand the surface morphology of the formulated adsorbent and to know the functional groups in the adsorbent not initially present in the MWCNT that may be responsible for the adsorption process.
2.4. CO2 Adsorption Experiment
- The CO2 adsorption experiments were performed using the experimental setup by Aimikhe and Eyankware [35]. Measured amounts of MWCNT-DES adsorbent were placed in the reactor immersed in a water bath for temperature control and dosed with the CO2 gas. Initial and final (equilibrium) pressures and corresponding temperatures were recorded. Initial pressure ranges were 2 and 15 psia at 26°C, 40°C, and 70°C. The corresponding adsorption capacity at each condition was also measured. Details of the experimental procedure can be found in the open literature [35].
2.5. Impact of CO2- Rich Adsorbent on Soil Properties
- The impact of CO2-rich MWCNT-DES and MWCNT-PEG on soil properties was determined. Soil properties like pH, amount of soil nutrients, and Total Organic Content (TOC) were investigated to know the effect of disposing of the CO2 –rich adsorbents into the environment. The test samples comprised the adsorbent + soil sample. The procedures for the analysis are described as follows:I. pH Determination: The pH meter of the Hanna 300 model was placed in each sample of 2g of dry sample mixed with 50 ml of distilled water and permitted to remain until stabilized. Reading was taken and recorded to determine the pH of the sample.II. Cat-ion Determination: 2g of dried sample was weighed into a 100ml beaker. 2ml HNO3 was added. The mixture was digested by heating, using a heating mantle. More acid was added, and digestion continued for 30-40 mins. Digestion was stopped when a clear digest was obtained. The flask was cooled, and the content was transferred into a 50ml volumetric flask through a Whatman No. 42, 150mm diameter filter paper, and the solution was made-up to the 50ml mark with de-ionized water. The resulting solution was analyzed for heavy metals using the Atomic Absorption Spectrophotometer (BULK SCIENTIFIC, 205).III. Total Organic Carbon (TOC) Determination. 0.2g of the sample was evaporated to dryness. Potassium permanganate and sulphuric acids were added in a ratio of 1:2, respectively, and mixed with distilled water after 30 mins. Drops of ferritin solution were added and titrated to endpoint.
3. Results and Discussion
3.1. Characterization of MWCNT-DES and MWCNT-PEG Adsorbents
- Results of the SEM images of MWCNT-DES and unmodified MWCNT using Flick through Electron Microscopy (SEM) showed that MWCNT possesses clogged pore spaces compared to the modified deep eutectic solvent. Figure 4 shows this result. Hence, the ability of MWCNT-DES to adsorb CO2 gas molecules on its surface is enhanced due to pore availability. However, the unmodified MWCNTs are densely clogged, making pores less available for CO2 adsorption. Figure 5 shows the FTIR spectra for the MWCNT-DES and MWCNT. For the MWCNT-DES adsorbent, the material can be described as a complex material with numerous adsorption peaks. From the spectrum, a broad adsorption band was noticed in the range of 3,250 to 3,650 cm-1, indicating the presence of the normal polymeric –OH group consistent with hydrogen bonding. Adsorption bands consistent with primary and secondary amines, open chain Imino (C=N-) group, triple bonds, C-H stretching of the ether functional group, carbonyl group, and amides were found to exist in the adsorbent. For the unmodified MWCNT, no broad adsorption band was noticed in the range of 3,250 to 3,650 cm-1, indicating the absence of the normal polymeric –OH group consistent with hydrogen bonding.
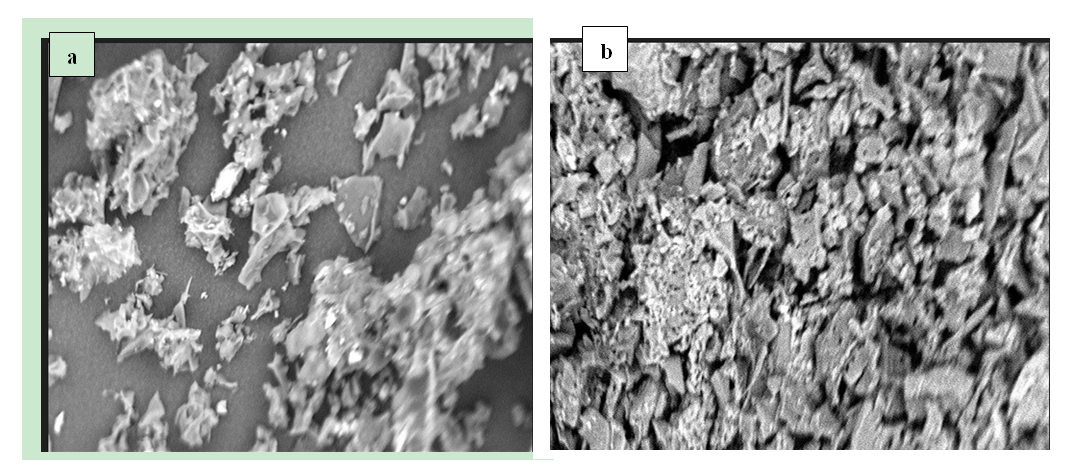 | Figure 4. SEM image of (a) MWCNT-DES and (b) unmodified MWCNT |
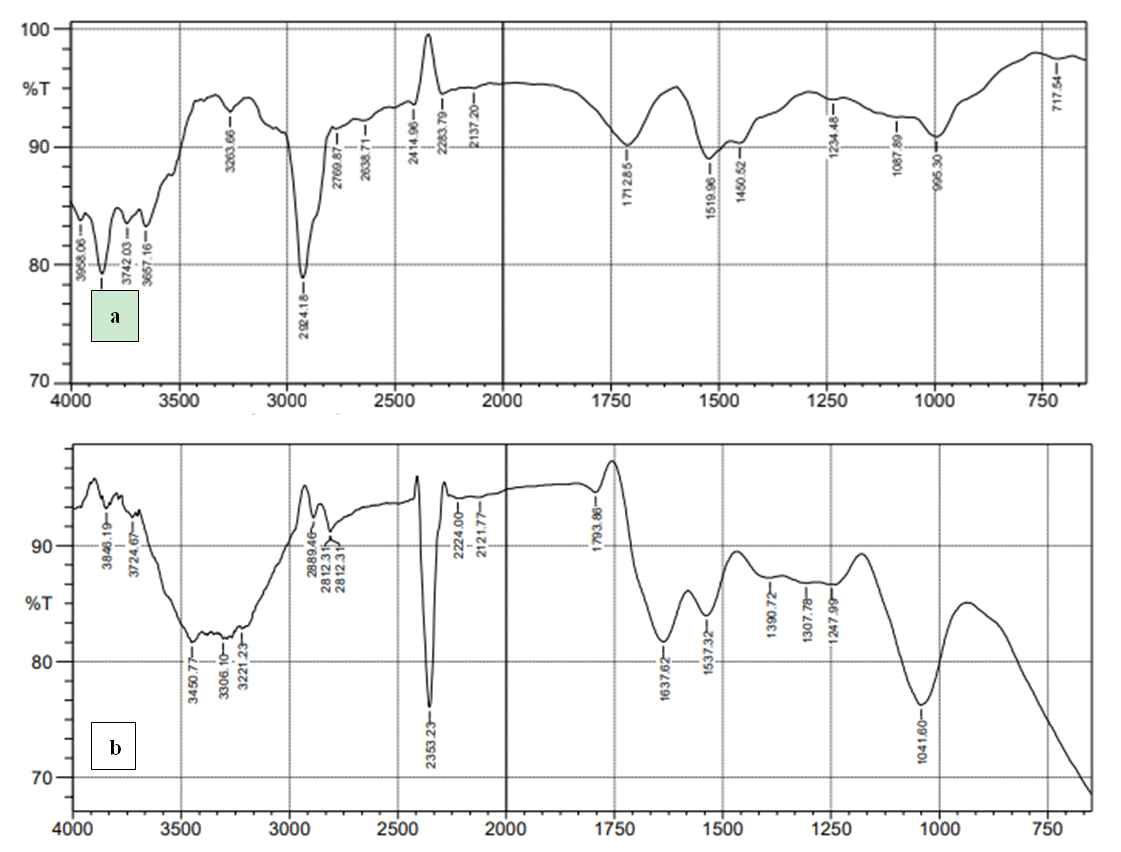 | Figure 5. FTIR spectra of (a) Unmodified MWCNT and (b) MWCNT-DES |
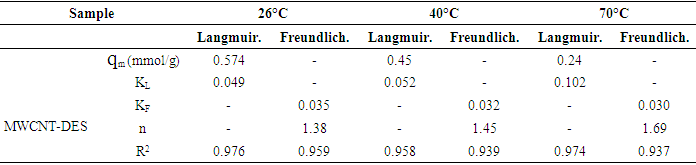
3.2. CO2 Adsorption Capacity of Adsorbents
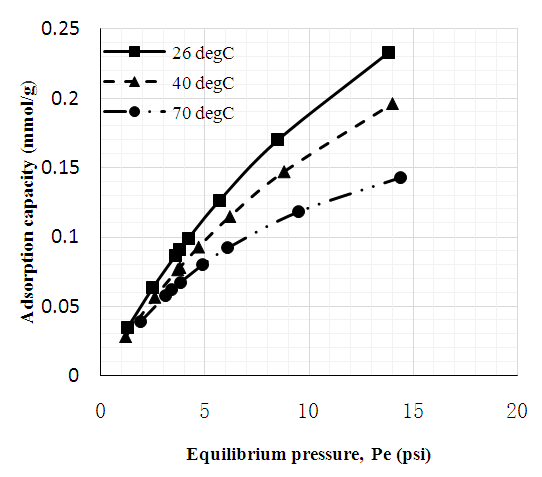
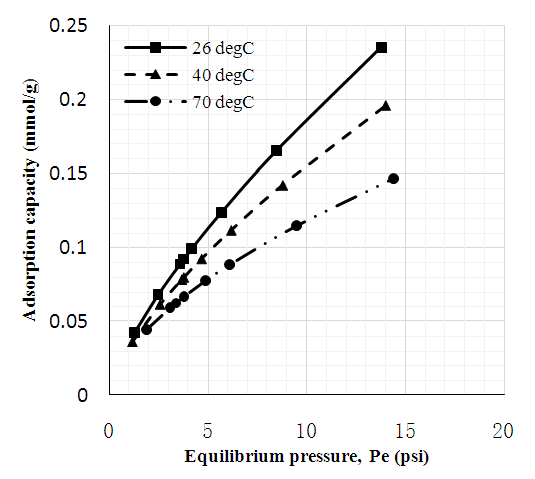
- Figures 6 to 8 showed that for CNT, MWCNT-DES, and MWCNT-PEG, CO2 adsorption capacity increased as pressure increased through 2 to 15 psi at 26, 40, and 70°C, respectively. This trend was also reported by Babaei et al. [37], wherein an increase in adsorption pressure corresponded with an increase in adsorption capacity at constant temperatures. For example, the adsorption capacity of CNT at 26°C increased through 0.082, 0.149, and 0.174 mmol/g at a P1 pressure of 2 to 15 psi. The same trend was noticed for MWCNT-DES and MWCNT-PEG at 26°C, where adsorption capacities increased through 0.087, 0.187, 0.226 mmol/g, and 0.063 0.180 and 0.22 mmol/g, respectively. The justification behind this noticed pattern is that, at increased pressures, CO2 molecules are impacted with a higher force that makes them assess the adsorbent's pores, meaning higher adsorbent adsorption capacities. This result agrees with the work of Kang et al. [38], which showed that the CO2 adsorption capacity of adsorbents is a component of strong gas mobility which is improved at increasing pressures.
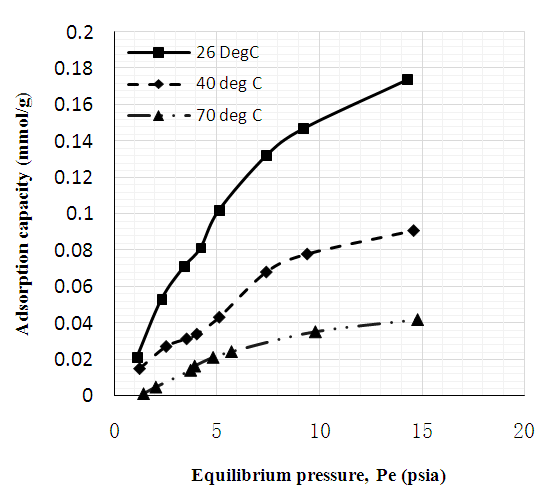 | Figure 6. CO2 adsorption capacity of unmodified MWC |
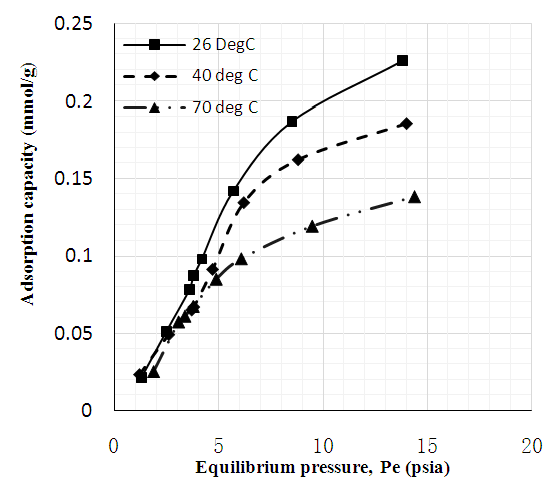 | Figure 7. CO2 adsorption capacity of MWCNT-DES |
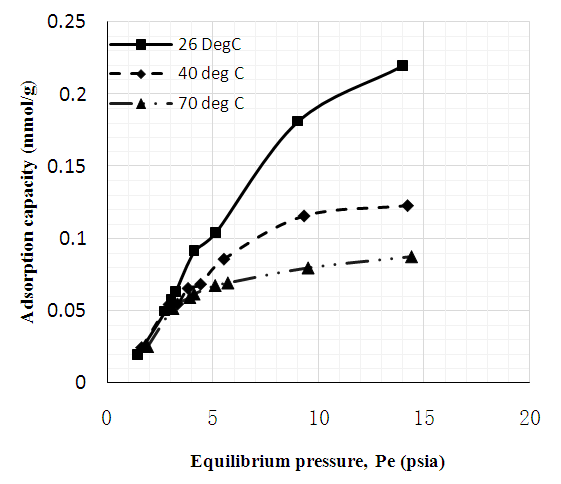 | Figure 8. CO2 adsorption capacity of MWCNT-PEG |
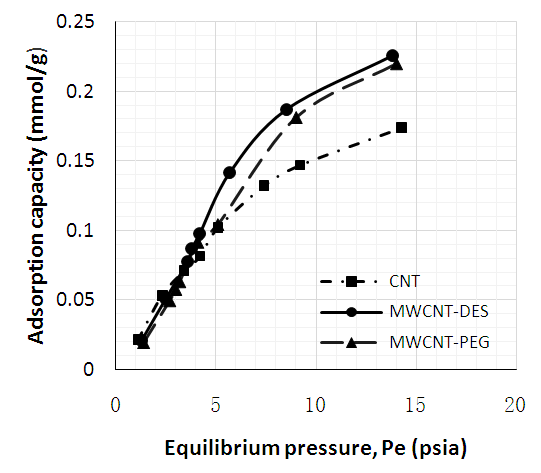 | Figure 9. Adsorption capacities of adsorbents @ 26°C |
|
 | Figure 10. Langmuir adsorption isotherm |
 | Figure 11. Freundlich adsorption isotherm |
3.3. Impact of CO2- Rich Adsorbent on Soil Properties
- An assessment of the effect of CO2-rich adsorbents on soil showed that MWCNT-PEG was soluble in water while MWCNT-DES was insoluble in water at ambient conditions. Herein, the control sample denotes the soil sample that does not contain CO2-rich adsorbents. In the results of the pH of samples, decreasing pH concentration through MWCNT-DES (7.6), MWCNT-PEG (7.5), MWCNT-PEG + control (7.5) and MWCNT-DES + control (7.2) were recorded. Although adding these CO2-rich adsorbents increased the acidity of the soil samples, their values were still within WHO limits of 6.5 – 8.5. Furthermore, it is apparent from the outcomes that the addition of MWCNT-PEG to the control did not affect the acidity of the control, unlike MWCNT-DES, highlighting its environmentally benign nature. Regarding TOC measured in mg/kg, adding MWCNT-DES (TOC Content of 3.16 mg/kg) to the control increased the TOC from 0.12 mg/kg to 3.77 mg/kg, highlighting its positive impact on soil nutrients. Similarly, adding MWCNT-PEG (TOC content of 2.92 mg/kg) to the control increased the TOC from 0.12 mg/kg to 2.90 mg/kg.The soil’s macronutrient analysis showed that the MWCNT-PEG increased the K+ concentration of the control from 4.18 ppm to 29.30 ppm, and MWCNT-DES increased the K+ concentration to 28.0 ppm, highlighting the positive impact of these CO2-rich adsorbents on the soil. On the Contrary, MWCNT-DES and MWCNT-PEG decreased the Mg2+ concentration of the control sample. MWCNT-PEG (76.29 ppm) decreased the Mg2+ concentration of the control from 99.21 ppm to 98.20 ppm, while MWCNT-DES (91.96 ppm) decreased the Mg2+ concentration of the control sample from 99.21 ppm to 95.42 ppm. Similarly, adding MWCNT-PEG and MWCNT-DES to the control reduced the Ca2+ concentration of the soil. As for MWCNT-PEG, a decrease in the Ca2+ concentration of the control from 23.40 ppm to 22.68 pm was observed, while for MWCNT-DES, a reduction in the Ca2+ concentration of the control from 23.40 ppm to 22.10 ppm was recorded accordingly. Figure 12 shows the soil test results.
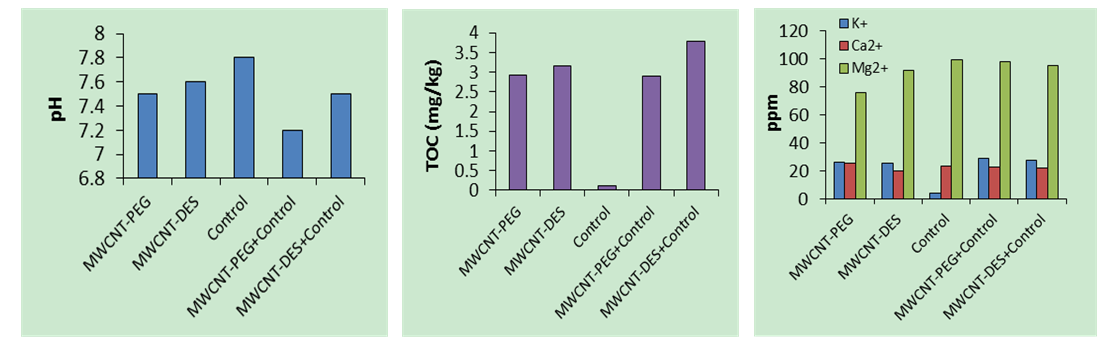 | Figure 12. Comparison of pH, TOC, and Ion concentrations |
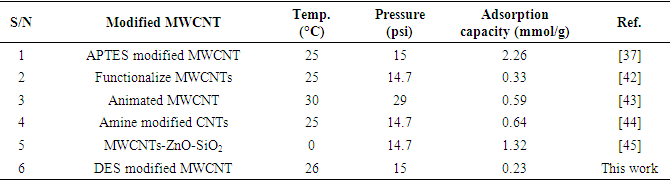
3.4. Comparison of Modified MWCNT to Similar Adsorbents in Literature
- The adsorption capacity of MWCNT-DES adsorbent at 26°C and 15 psi was compared to other published MWCNT-modified adsorbents. As emphasized in Table 3, MWCNT-DES showed limited CO2 adsorption capacity compared to similar adsorbents. In this view, MWCNT-DES is not a suitable adsorbent for CO2 capture at post-combustion conditions.
|
4. Conclusions
- This study investigated the CO2 adsorption efficiency of MWCNT modified with choline chloride + glycerol deep eutectic solvent. A CO2 adsorption capacity of 0.226 mmol/g at 26°C and 15 psi was observed for the adsorbent. This adsorption capacity increased with pressure and decreased with increasing temperature. Compared to an MWCNT modified with polyethylene glycol (MWCNT-PEG), results showed a relatively similar adsorption capacity of 0.220 mmol/g compared to MWCNT-DES (0.226 mmol/g). The adsorption process of the MWCNT-DES was a typical physisorption process best described by the Langmuir model. In the event of disposal to the environment, soil tests revealed that the CO2-rich MWCNT-DES adsorbent is environmentally friendly and has the propensity to improve soil properties. However, due to its low adsorption capacity (0.226 mmol/g at 26°C and 15 psi), the formulated MWCNT from acetylene via chemical vapor deposition and modified with deep eutectic solvent prepared from choline chloride and glycerol is not a suitable adsorbent for CO2 capture at post-combustion conditions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML