-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2021; 11(1): 1-8
doi:10.5923/j.ijee.20211101.01
Received: Apr. 5, 2021; Accepted: May 12, 2021; Published: Jul. 26, 2021

Effect of Methyl Tertiary Butyl Ether (MTBE) and Ethyl Tertiary Butyl Ether (ETBE) on the Properties of Gasoline
Theophilus Kwesi Shiako, Benjamin Afotey
Department of Chemical Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Correspondence to: Theophilus Kwesi Shiako, Department of Chemical Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Crude Distillation Unit often produces gasoline of low Research Octane Number (RON). For efficient engine performance, high RON-gasoline is required. Refiners resort to the use of different chemical compounds called additives to improve the RON of gasoline. One group of these additives is fuel oxygenates, oxygen-containing hydrocarbons. In this study, experimental results of the effect of two fuel oxygenates, Methyl tertiary Butyl Ether (MTBE) and Ethyl tertiary Butyl Ether (ETBE) on the RON, RVP, Distillation, Density, Oxidation Stability (Induction Period), Washed Gum and Copper corrosion were examined and compared. The results showed that ETBE improves the RON of gasoline slightly better than MTBE. The increase in RVP of the gasoline was higher with MTBE than ETBE. Distillation curves were not significantly different from each other and other properties such as oxidation stability and copper corrosion were the same for the blends used.
Keywords: Research Octane Number (RON), Fuel Oxygenates, MTBE, ETBE, Octane Booster
Cite this paper: Theophilus Kwesi Shiako, Benjamin Afotey, Effect of Methyl Tertiary Butyl Ether (MTBE) and Ethyl Tertiary Butyl Ether (ETBE) on the Properties of Gasoline, International Journal of Energy Engineering, Vol. 11 No. 1, 2021, pp. 1-8. doi: 10.5923/j.ijee.20211101.01.
Article Outline
1. Introduction
- Since the advent of the Spark Ignition (SI) engine, one challenge that has persisted is the quality of fuel used. Gasoline, which is the fuel mostly used in SI engines, combusts rapidly if its chemical structure make-up is straight-chain hydrocarbons and causes knocking in the engine [1,2]. Knocking reduces the lifetime of engines significantly and should be avoided as much as possible [3]. The tendency of an engine to knock or not is determined by the octane number (ON) of the fuel being used [4]. Gasoline with high octane rating can withstand high compression. Thus, SI engines can be designed with higher compression ratios and boost levels (for turbocharged engines) which could lead to higher engine and vehicle efficiencies. Higher efficiencies would translate to reduced fuel consumption and greenhouse gas (GHG) emissions over time [5–7].Using refinery processes to increase the ON of gasoline is very expensive. This led to the search for octane enhancing additives (octane boosters) for gasoline. Tetra Ethyl Lead (TEL) was the first to prove successful and was used for several years until health concerns and vehicle catalyst poisoning were raised over lead (Pb) deposits [8,9]. Subsequently Ethyl Corporation discovered Methylcyclopentadienyl Manganese Tricarbonyl (MMT) as a suitable cost-effective substitute for TEL. Again, public health concerns were raised over effect of manganese in the environment which is released upon combustion of gasoline containing MMT [8,10–12]. Similar health concerns were raised over other octane boosters such as dicyclopentadienyl iron (commonly referred to as ferrocene) [10,13].These octane boosters cause similar health concerns because they all have similar structures. They are organometallic compounds whose metals are released into the environment upon combustion [8,10]. These metals in the atmosphere are easily inhaled by humans resulting in health concerns. For instance, when fuel containing MMT is combusted, manganese vapours released in the air is inhaled, enters directly into the bloodstream through the lungs without going through the digestive system to be regulated by the liver. The metals get deposited in the brain leading to severe health condition called manganism [10,14,15].Automakers have raised concerns over the use of these organometallic octane boosters. There are concerns of metal deposits on sensitive vehicle parts such as on spark plugs and catalytic converters in modern emission control systems [11,16]. These concerns necessitated the search for non-metal-based octane boosters. Fuel oxygenates have been found to be suitable replacements. Fuel oxygenates are organic compounds containing oxygen which are added to fuels (mostly gasoline) to improve the oxygen content of the fuel. They are known to improve the octane rating of base gasoline [8,17]. Common fuel oxygenates used around the world are alcohols such as ethanol and methanol and fuel ethers including Methyl tertiary butyl ether (MTBE), Ethyl tertiary butyl ether (ETBE) and Tertiary amine methyl ether (TAME) [18].Currently there is a worldwide ban on TEL. In United States of America (USA), Europe and other developed countries, MMT is banned and fuel oxygenates are used as octane boosters [15,16]. Developing countries, including Ghana, still use gasoline containing MMT for two main reasons; firstly, it offers less expensive fuel for the same octane rating as that with fuel oxygenates and secondly, there are no strict environmental regulations which could lead to ban of MMT.MTBE and ETBE are ethers that are used as oxygenates in gasoline. MTBE (C5H12O) has molecular weight of 88.15, of which 18% is oxygen. It is a colourless liquid with a strong, terpene-like odour and taste in water. Its melting and boiling points are -109°C and 55.2°C respectively. It has a high vapour pressure of 245 mmHg @ 25°C, water solubility of 42 g/L @ 20°C and is very soluble in hydrocarbon solvents. ETBE (C6H14O) has molecular weight of 102.18, of which about 16% is oxygen. ETBE is a colourless to light yellow liquid. Its melting and boiling points are -94°C and 71°C respectively. It has a vapour pressure of 210 mmHg @ 25°C, water solubility of 12 g/L @ 20°C and is very soluble in hydrocarbon solvents [19–22]. Both ethers are not as biodegradable as the hydrocarbons present in natural gasoline [23,24]. A study by [25] in anticipation of the European 2005 regulations concluded that the use of oxygenates in Reformulated gasoline reduced THC, NOx and particulate emissions. Analysis performed by [26] indicate a net positive effect of fuel oxygenates on total GHG emissions. [27] report a slight increase in engine efficiency when fuel oxygenates are blended into gasoline in addition to the advantages. [28] in his paper concludes that substituting MTBE for the high aromatic gasoline blend reduces the level of NOx and CO emissions, thus improving air quality and the state of the environment. His study revealed a decrease in CO emissions when MTBE is added to gasoline; decreasing CO emissions with increasing MTBE content. Mutagenicity of gasoline is reduced when MTBE and ETBE are added as octane boosters in place of higher aromatic content. Reduced aromatic content results in reduced emissions of toxic Polycyclic Aromatic Hydrocarbons (PAH) and 1,3-butadiene which are responsible for mutagenic effects [29,30].Using MTBE and ETBE in place of organometallic additives eliminates the risk of metal of deposits on vital engine components (for example spark plugs and valves) and advanced emission systems such as catalyst converters. This would translate to lower maintenance cost and longer life span.Ethers however have the disadvantage of generally decreasing the brake thermal efficiency of gasoline which results in increased brake specific energy consumption. This is effected on consumers by a slight increase in fuel consumption [7,31]. They are also generally costly to blend in gasoline compared to the organometallic compounds [2,24].Ethers are highly oxidizable in air forming explosive peroxides [32]. In gasoline however, their oxidizing ability is significantly reduced hence peroxide formation is almost non-existent. For long periods of storage (over 2 years), addition of antioxidants is recommended as a precautionary measure [33,34].
2. Materials and Methods
- ASTM test methods were used for the various analyses in this study. Base gasoline was obtained from the Tema Oil Refinery, with a Research Octane Number (RON) of 81.3. 1-litre samples each of 1.0, 1.5 and 3.0%v/v MTBE and ETBE blends were prepared. The prepared samples were labelled M1.0, M1.5, M3.0 for 1.0, 1.5, 3.0%v/v MTBE and E1.0, E1.5, E3.0 for 1.0, 1.5, 3.0%v/v ETBE respectively.All 6 samples and the base gasoline were kept in the refrigerator for about an hour to reduce loss of lighter hydrocarbons during the analyses.
2.1. Research Octane Number (RON)
- The RON values of the samples were determined by the Cooperative Fuel Research (CFR) engine. The CFR engine consists of a RON determination unit, a box-type crankcase with flywheel connected by V-belts to power an electrical motor, an adjustable compression ratio of 4:1 to 18:1, a valve mechanism to provide constant valve clearance as compression ratio changes, a piston, a fuel system consisting of a carburettor of single vertical jet and fuel flow control to permit adjustment of air-fuel ratio, and an ignition which electronically triggers condenser discharge through coil to spark plug.Each sample was run to determine its knock intensity (KI). Two reference fuels of known RONs, one higher and the other lower, were then run to obtain KIs higher and lower than the KI of the sample respectively. The RON of the sample was calculated by interpolation [35].
2.2. Reid Vapour Pressure (RVP)
- The Stanhope-Seta of model number 81000-2U was used to perform this analysis. The equipment has a thermostatically controlled test chamber with a moveable piston that expands the volume after sample introduction. 3 ml of the refrigerated, air-saturated test specimen was injected into the evacuated test chamber. The total volume of test sample injected was about a fifth of the internal chamber volume. The test specimen attained thermal equilibrium at the test temperature, 37.8°C (100°F) in the chamber. The pressure transducer sensor and indicator measured the total pressure, which is the sum of the partial pressure of the sample and the partial pressure of the dissolved air. The measured total vapor pressure was converted to a dry vapor pressure equivalent (DVPE) which was recorded as the RVP of the sample [35].
2.3. Atmospheric Distillation
- The ASTM method D86 was followed and the manual atmospheric distillation apparatus Herzog HDA 620 was employed. For each analysis, 100mL of the sample was placed in a round-bottom flask and heated at a rate specified for a sample with its vapour pressure characteristics. A thermometer was inserted into the flask to measure the temperature of emanating vapour. At the first drop of condensate, the vapor temperature was recorded as the initial boiling point (IBP). The vapour temperatures were recorded at 5, 10, 20, 30… 90 and 95% distilled volumes with the aid of a 100 ml measuring cylinder. The final temperature, recorded as the end boiling point (EBP), is at 99% distilled volume [36].
2.4. Density @ 15°C
- Densities of all fractions were measured following ASTM method D1928. The fraction was brought to a specified temperature and a test portion transferred into a cylindrical container that had been brought to approximately the same temperature. The appropriate hydrometer, also at similar temperature, was lowered into the test portion and allowed to settle. After equilibrium temperature was reached, the hydrometer scale was read and the temperature of the test portion taken. The observed hydrometer reading was reduced to the reference temperature at 15°C [36].
2.5. Copper Corrosion
- The copper corrosion test was used to determine the corrosiveness of gasoline to copper. ASTM D130 test method was used. 30 ml of the samples were placed in a test tube. The surfaces of the copper strips were then prepared by rubbing against silicon carbide moistened with a wash solvent (isooctane). A final polishing step was done with silicon-carbide grains on absorbent cotton while holding the strips in a polishing vice. Each polished copper strip was immediately immersed in the test tube containing the sample. The test tubes were carefully slid into test bombs and closed tightly. The bombs were then placed in a water bath at 40°C for 3 hours. The bombs were then taken out, contents emptied carefully and copper strips withdrawn with a pair of stainless-steel forceps. The copper strips were washed and compared with the ASTM Copper Strip Corrosion Standards of 1a, 1b 2a-e, 3a-b and 4a-c with 1a showing the least corrosion effect and 4c the maximum corrosion effect [36].
2.6. Washed Gum Content
- The test method for this analysis was ASTM D381 for determination of existent gum in fuels by jet evaporation. Beakers were first conditioned. The conditioning involved washing with equal volumes of gum solvent (toluene-acetone mixture), rinsed with water and immersed in a mildly alkaline detergent cleaning solution for 6 hours. The glassware was removed with a pair of stainless-steel forceps, dried in an oven at stable temperature of 150°C for an hour and transferred into a cooling vessel for about 2 hours to attain room temperature.The conditioned beakers were weighed, recorded and filled with 50ml of each sample. The beakers were then transferred into the air and steam jet tester and the conical jets placed in each beaker. The jet tester takes 4 samples and a tare, as control, at every run. The tare is also a conditioned beaker but without any sample. The samples were heated with air (no steam) and allowed to evaporate under controlled conditions for 30 minutes at 159°C. The beakers were transferred to a desiccator for about 2 hours to cool to room temperature. The unwashed gum was extracted with 25 ml of heptane. The extraction process was repeated to obtain the washed gum.The beakers were dried in the evaporating bath at 159°C for 5 minutes and cooled in the cooling vessel for 2 hours. The beakers and the tare were weighed and recorded. The tests were repeated and the average results reported in mg/100 ml as the washed gum content. The solvent washed gum content of each sample was calculated using the equation:S = 2000 (C – D + X – Z)S – solvent washed gum content, mg/100 mLC – mass recorded for the sample beaker plus residue in (g)D – mass recorded for the empty sample beaker, gX – mass recorded for the tare beaker before analysis, gZ – mass recorded for the tare beaker after analysis [35]
2.7. Oxidation Stability (Induction Period)
- PetroOXY from petrotest© was used to determine the oxidation stability (induction period) of the gasoline blends using ASTM D525. The equipment is connected to an oxygen supply gas. The pressure of the oxygen supply was set to 700 kPa. The test chamber was cleaned with dry tissue, filled with 5ml of the sample to be tested and then tightly closed. The sample was combined with oxygen at a pressure of 700 kPa at 25°C and then heated to 140°C for 20 minutes. The pressure drop within the chamber was then recorded to determine the oxidation stability of the sample.
3. Results and Discussion
- The properties of the samples are compared with the Ghana Standard. A summary of the results is presented in Table 1.
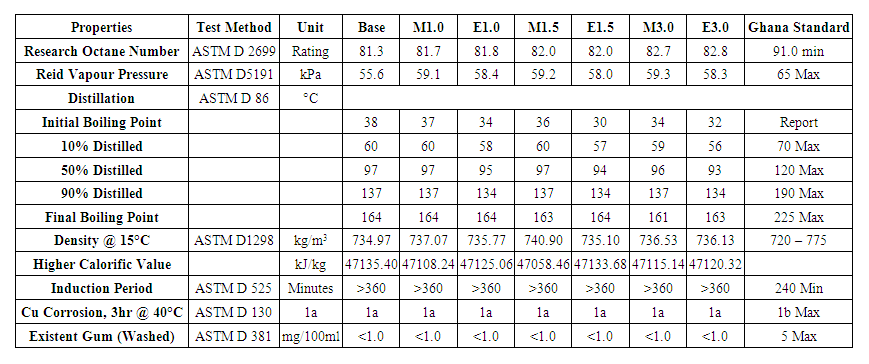 | Table 1. Summary of results |
3.1. Research Octane Number
- The quality of gasoline is often determined by its octane number. This property of gasoline is very important to ensure consumers are satisfied that the fuel they use in their vehicles meets the engine requirement for compression rate achieved without self-igniting. The RON evaluates the gasoline’s resistance to self-ignition under mild working conditions with low rotations of the engine (600 rpm). The Motor Octane Number (MON) indicates the gasoline’s antiknock ability under more severe working conditions and at high rotations (900 rpm). RON simulates city drivability conditions while MON simulates highway drivability conditions. The antiknock index (AKI) is the arithmetic mean of the RON and MON. In this study, only the RON values were determined with the CFR engine in accordance with the test method ASTM D 2699.From the experimental results, it can be observed that MTBE produces an average of 0.46 RON increase for each volume percentage addition to base fuel of 81.3 RON compared to 0.49 for ETBE. This implies ETBE has a slightly better octane response than MTBE. The increase in RON is also observed from Figure 1 to follow a linear trend with their high R2 values. This is in agreement with findings of Da Silva et al. (2005) on the addition of oxygenates to two different base gasolines; 98 and 82 RON base gasolines. In both cases, ETBE showed a slight advantage over MTBE for the same oxygenate concentrations.
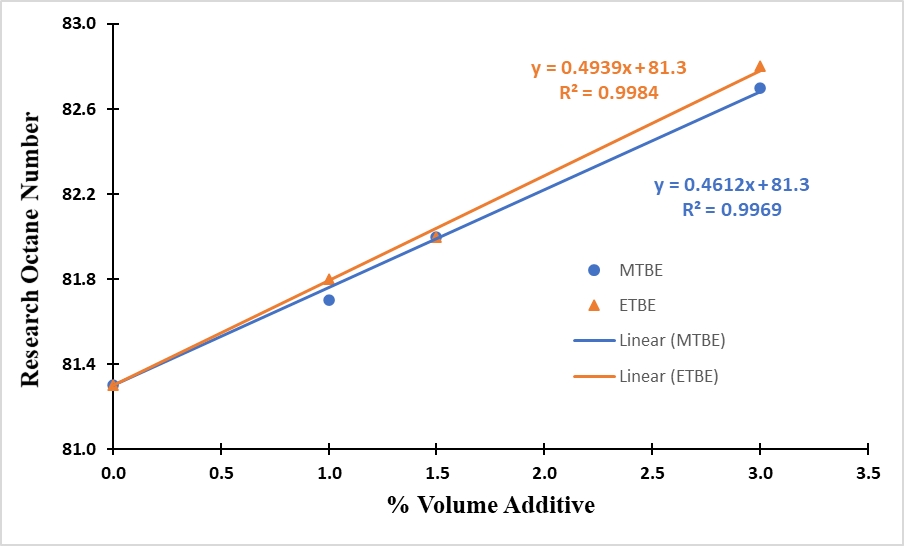 | Figure 1. Comparison of RON Response of MTBE and ETBE Blended Gasoline |
3.2. Reid Vapour Pressure
- Reid Vapour Pressure (RVP) is the vapor pressure of gasoline measured at 37.8°C. RVP, together with boiling range of gasoline govern ease of starting, engine warm-up, rate of acceleration, loss by crankcase dilution, mileage economy, and tendency toward vapour-lock [18]. This test provides a good indication of the volatility of the gasoline’s lighter portion and serves to evaluate the tendency of a gasoline to evaporate, so that the higher the vapor pressure, the more easily the gasoline evaporates. A very high vapor pressure may lead to plugging of the fuel flow caused by the gasoline vapours blocking the line and preventing the fuel from being pumped to the injection valves. Moreover, fuels with high vapor pressures present high volatile organic compounds emission rates [38].Addition of MTBE and ETBE to the base gasoline raises the RVP of the gasoline. The rate of RVP increase is initially high but reduces as the fractions of MTBE and ETBE increase. Though RVPs of pure MTBE (53 kPa) and ETBE (32 kPa) are lower than the RVP of the base gasoline (55.6 kPa), their addition raises the RVP of the obtained blends. This could be attributed to intermolecular interactions between the gasoline and the ethers used in this study. The profiles observed in Figure 2 indicate that MTBE has a higher effect on RVP of gasoline than ETBE.
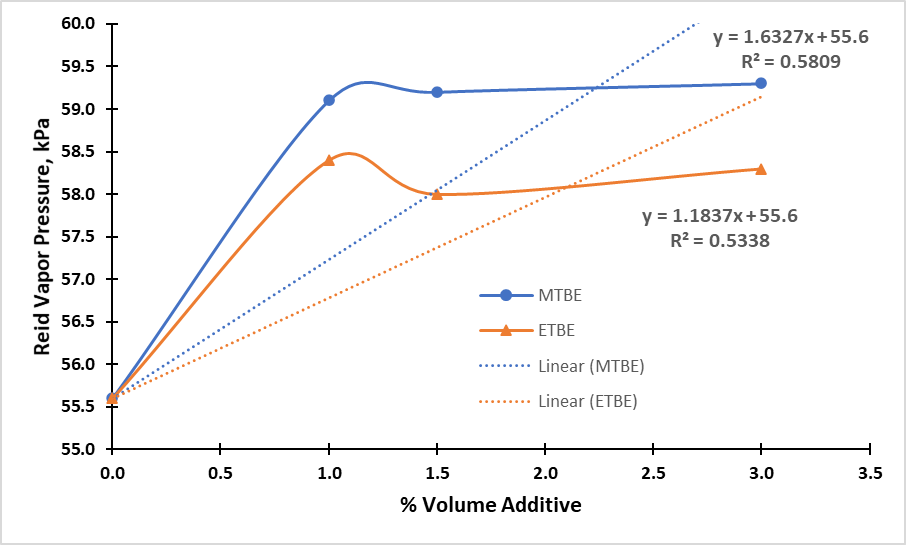 | Figure 2. Comparison of RVP Response of MTBE and ETBE Blended Gasoline |
3.3. Atmospheric Distillation
- Engine behaviour is affected significantly by distillation curve profile. The vapour lock, evaporative losses from the tank, and hot or cold engine starting depend on lighter fractions (0% to ~ 40% of distilled volume). Medium fractions (~40% to ~80% of distilled volume) affect the ice formation in intake air system, the engine warm-up, the vehicle acceleration and the short-trip fuel economy. Lastly, the long-trip fuel economy, the oil dilution, VOC and the formation of combustion deposits depend on heavy fractions (~80% to 100% of distilled volume). The distillation curve can, in simple terms, be represented by three points: T10, T50 and T90, which represent the temperatures at which 10, 50 and 90% vaporization of the gasoline’s initial volume occurs. These temperatures characterize the volatility of the fuel’s light, medium and heavy fractions. It is observed that the initial boiling point (IBP) decreases as the concentrations of both MTBE and ETBE increases. This observation could be associated with the increased RVP obtained which is an indication of increased volatile content. The IBP of the blends with ETBE were observed to be lower than those of MTBE and this is attributable to the higher volatility of ETBE. The addition of MTBE to the base gasoline had relatively no effect on T10. The T10 values were relatively constant for all three MTBE blends. However, T10 values of ETBE showed a decreasing trend with increasing ETBE volume. The 1.0% ETBE blend was 2°C lower than the base gasoline and 1°C lower than the 3% MTBE blend. Thus, ETBE has a greater impact on the volatility of light fractions in the base fuel than MTBE. Similar observations were made at both T50 and T90 which is a confirmation of the impact each of the ethers has on the volatility of the base gasoline. The Final Boiling Point (FBP) values reduced slightly for the MTBE blends but remained relatively constant for all the ETBE blends.From Figure 3, it can be observed that all the distillation curves of the MTBE blends are similar to the that of the base gasoline. However, a small deviation can be observed at 80% distillation for the ETBE blends; the deviation is observed to increase as the ETBE fractions increase from 1% to 3%.
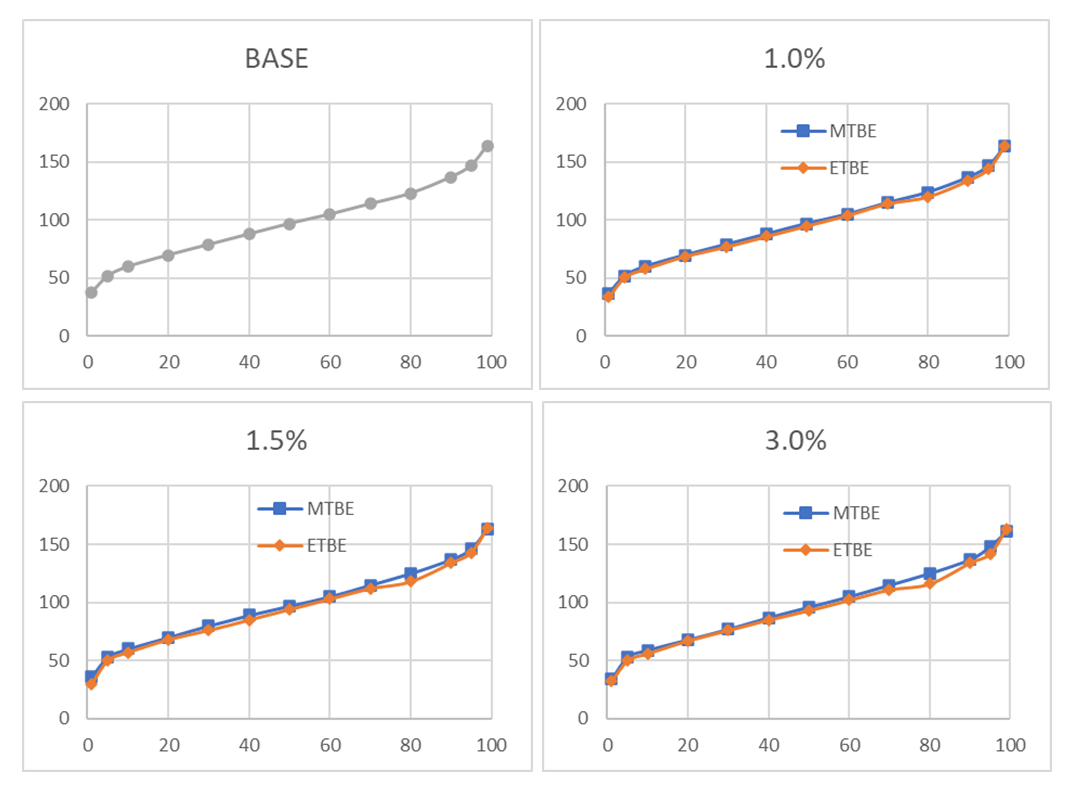 | Figure 3. Comparison of Base, 1.0%, 1.5% and 3.0% Blended Gasoline Distillation Curves |
3.4. Copper Corrosion
- Despite the removal of most sulphur content during refinery of crude petroleum, some of the remaining sulphur compounds present in petroleum products could have corrosive effect on various metals. However, corrosivity is not always directly associated with the total sulphur compounds present. The effect can vary depending on the types of sulphur compounds in the product.Fuels with high copper corrosivity could cause problems in storage, pipelines and copper tubes in vehicles. Corrosion in gasoline storage tanks could lead to contamination of gasoline and significant changes in the physicochemical properties of the gasoline. The use of such products would result in lower engine performance, increased fuel consumption and damage to engines. Also, maintenance cost for storage equipment could increase significantly when the corrosivity of gasoline is high. Similar debilitating effects would result in pipelines and copper tubes.From Table 1, the addition of 1.0%, 1.5% and 3.0% of MTBE and ETBE to the base gasoline used in this test had no effect on the corrosivity of the blends. As with the base gasoline, all the blends obtained a 1a rating which is designated slight tarnish according to the ASTM Copper Strip Classifications.
3.5. Density @ 15°C and Higher Calorific Value
- It is generally observed that the addition of MTBE and ETBE increased the density of the base gasoline, which had a density of 734.97 kg/m3. The increase in density was observed to be higher for the MTBE blends than the ETBE blends because of the higher density of MTBE (750 for MTBE and 743 for ETBE at 20°C). In contrast to what most researchers report [39,40], there was no linear trend for the increase in density. This anomaly is likely due to loss of lighter hydrocarbons during sample preparations and analyses. The probable loss of lighter hydrocarbons was highest in 1.5% MTBE blend. Another possible reason for this outcome is the molecular interactions between the additives and the base gasoline which results in the formation of a non-ideal solution. A non-ideal solution does not usually follow a linear trend even though its outcome could be predicted.The density of gasoline has a slight effect on its heating (calorific) value and consumption rate. Energy content of the fuel is based on mass while purchase is based on volume. Two gasolines having the same energy content but different densities will have different energy densities which would imply different consumption rates. For example, assuming the density of Gasoline A is 730 kg/m3 and that of Gasoline B is 735 kg/m3 but both have the same heating value of 40 MJ/kg; their energy densities will be 29.2 GJ/m3 and 29.4 GJ/m3 for Gasoline A and Gasoline B respectively. If the energy demand of an engine is 100 GJ, 3.42 m3 of Gasoline A and 3.40 m3 of Gasoline B will be required. At an assumed selling price of GHS 100.00 per m3, it will cost GHS 342 to run the engine with Gasoline A and GHS 340 with Gasoline B. Thus, using a higher-density gasoline may result in a slightly higher calorific value per unit and consequently a lower cost of fuelling. This is however not often the case because of the dependence of heating value on the density of gasoline.The higher calorific value (HCV) can be estimated by the equation [41,42];HCV, cal/g=12,400 – 2100 (sp. gravity)2The estimated HCVs are presented in Table 1. The HCVs decreased for all the blends in comparison with the base gasoline which is a direct consequence of the increasing density when MTBE and ETBE are added to the base gasoline. In actual bomb calorimetry test, the HCVs of both the MTBE and ETBE blends will be expected to decrease not only as a result of the decrease in density but also due to the lower HCVs of the additives compared with that of the base gasoline.The calorific value of gasoline has an impact on the fuel consumption and consequently the cost of fuel to consumers. In a scenario where the calorific values of two gasolines are 40 MJ/kg and 45 MJ/kg, for Gasolines A and B respectively; the energy densities will be 29.2 GJ/m3 and 32.9 GJ/m3 for Gasolines A and B respectively. For a 100 GJ engine, 3.42 m3 of Gasoline A and 3.04 m3 of Gasoline B will be required. At an assumed selling price of GHS 100.00 per m3, it will cost GHS 342 to run the engine with Gasoline A and GHS 304 with Gasoline B. Hence, it will cost lower to run the engine with a higher HCV-gasoline than with a lower HCV. This observation is however not absolute in practice because the actual energy output is dependent on other factors such as fuel composition and operating conditions.
3.6. Oxidation Stability and Washed Gum Content
- Problems associated with retention of fuel quality arise when petroleum products are being transported over long distances as well as blending and transfusion of the products many times in the transport terminals. The speed with which (unsaturated) hydrocarbon molecules in contact with oxygen may undergo oxidation is determined by the factor of fuel oxidation instability. The oxidation stability of a petroleum product is commonly determined by the induction period. The induction period indicates the tendency of gasoline to develop gum during storage.Gum content of a fuel is the non-volatile part of the fuel. When the hydrocarbons in gasoline react with atmospheric oxygen or with each other over a period, the gasoline’s physical and chemical properties change. As the reaction proceeds, non-volatile high molar mass molecules (gum) could be formed and deposited along vehicle fuel systems. Gum deposits could build up in the carburettor and admission valves, hence retarding the efficiency of air-fuel mixture leading to deficient combustion. Similar problems are faced in the injection nozzles and plugs in electronic injection cars. Gum deposits adversely affect drivability (engine choking, hesitation), engine performance (power loss, reduced acceleration, increased fuel consumption, detonation), and exhaust gas emissions (CO, NOx, etc.). The vital vehicle components that get blocked by gum would have to be maintained more regularly over time if the gum content of the fuel used is high. This would translate into higher maintenance costs and reduced lifetime of the components. There could also be gum formation in storage tanks when fuels are kept for a long period even at normal conditions. Gum formation is mainly dependent on gasoline composition, origin, type of refinement, and storage room conditions.All the samples recorded induction periods of >360 minutes which is an indication that the fractions of MTBE and ETBE added to the base gasoline do not adversely affect the oxidation stability of the blends. This observation is in agreement with the non-formation of peroxides observation made by [33,34]. All samples yielded negligible washed gum content (<1.0 mg/100ml). This observation could be attributed to the properties of the fuel ethers. Under oxidising conditions, both ethers yield several similar products, albeit in different quantities. These include alkanes (methane, ethane), alkenes (ethylene, propylene, butenes), alcohols (methanol, ethanol, t-butyl alcohol), aldehydes and ketones among others. All these oxidation products do not form gum and hence the results [32,43]. The oxidation stability of the products as determined earlier could be a major contributor to the results obtained here. MTBE and ETBE have high volatilities and thus evaporate more easily than that of the base gasoline. Though their densities are slightly higher, they have lower molecular mass than that of the base gasoline used. Another factor could be the purity (99%) of the additives used. As observed by [44], the presence of contaminants (such as copper) in an additive could lead to increased gum content.
4. Conclusions and Recommendations
- Addition of MTBE and ETBE to natural gasoline improves the octane rating of gasoline. ETBE is observed to have a slightly better octane boost than MTBE, albeit at a significantly higher cost. For all the concentrations used in this study, other properties of gasoline met the minimum standards specified by the Ghana Standards Authority (GSA).It is recommended that the GSA, in collaboration with the Environmental Protection Agency, specifies oxygen content of gasoline to guide the use of fuel oxygenates as gasoline blends in Ghana. This step could reduce toxic emissions from vehicles and protect health of citizenry and the environment.
ACKNOWLEDGEMENTS
- The authors express their profound gratitude to Mr Godwin Amon and the entire hardworking staff of the Tema Oil Refinery Quality Control Department for their assistance in data collection. Special thanks also go to Baba Akaribo for his support in this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML