Everlyne K. Mosomi, Alex M. Muumbo, Reuben O. Marwanga
Department of Mechanical and Mechatronic Engineering, Technical University of Kenya, Nairobi, Kenya
Correspondence to: Everlyne K. Mosomi, Department of Mechanical and Mechatronic Engineering, Technical University of Kenya, Nairobi, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Carbon capture is immensely articulated as a technology that is centred on limiting the emission of CO2, which is linked to global climate change. Moreover, the need to attain a sustainable energy supply is viewed as being one of positive ways that will help to react with the increasing global national and local environmental issues. Likewise, Biogas being a renewable energy purifying it is both beneficial to the environment and society as a whole. The goal of this research is to develop a system that can scrub carbon dioxide as well as analyse, assess different materials used to capture CO2. Biogas is a gaseous mixture of approximately 45%-75% methane, 25%-55% carbon dioxide and 2000 PPM hydrogen sulphide depending on the biomass used. The biogas performance is seemingly affected by increasing CO2; thus it has to be removed before it causes more harm to the quality of Biogas produced. Besides it leads to lowering methane combustion performance, difficulties in biogas storage and transportation and reduction in calorific value of the biogas. In this work chemical absorption method was used to remove CO2 from biogas by using sodium hydroxide solution. Different concentrations of sodium hydroxide were used in cleaning biogas the concentrations include; 0.5molar, 1molar, 1.5molar and 2molar. Results showed that as molarities of NAOH were increased reduction of CO2 also increased. Experiments revealed the highest removal efficiency had been achieved at 2molar of NaOH absorbent solution reduction of CO2 achieved was 27.26% from 33.90% as initial value and maximum absorption was achieved when methane increment was about 30.85% from its 65.10% initial value. Gas obtained after removing CO2 from biogas resulted into increased methane content nearer to natural gas. Future works ought to centre their focus on the possibility of converting the sought residue and all the spent solutions in gaining all the value added products for small scale production of biogas.
Keywords:
Carbon dioxide, Biogas, Sodium hydroxide
Cite this paper: Everlyne K. Mosomi, Alex M. Muumbo, Reuben O. Marwanga, Design, Fabrication and Testing of a Carbon Dioxide Scrubber from Biogas, International Journal of Energy Engineering, Vol. 10 No. 4, 2020, pp. 117-122. doi: 10.5923/j.ijee.20201004.03.
1. Introduction
High concentrations of carbon dioxide in the atmosphere have led to global warming and climate change to world in general. In Kenya there is little technical capacity which has been done to mitigate CO2 emissions from the source. There are many poor communities in the African community who need to embrace the use of renewable energy technologies [1]. In such a situation, Kenya is not an exception. This research was focused on small scale scrubbing of the CO2 that is attained from biogas, with a view to enhancing the entire calorific value of the biogas. Biogas is a by-product of anaerobic decomposition of organic wastes due to its decomposition carbon dioxide is present as one of the constituents due to absence of oxygen. Two way of digestions include dry or wet, mostly wet digestion is preferred for processing organic waste except for cases where the substrate has high total solids content [2]. An induction to various elevated concentrations of CO2 can bring more risks to one’s life and even to climate change [3]. Atmospheric carbon dioxide concentrations have steadily increased. Notably, the anthropogenic contributes to increase of CO2 that brings negative effects to the environmental consequences has immensely led to the sustainability as well as carbon capture [4]. Global warming and climate change concerns have triggered global efforts to reduce the concentration of atmospheric carbon dioxide. Carbon dioxide capture is considered a crucial way for meeting CO2 emission reduction targets [5]. Biogas is widely considered as a suitable source of fuel for heating facilities, power generation and vehicles. However the total electrical efficiency decreases with decreasing CH4 content in biogas fuel [6]. The decrease of methane content is led by other constituents in biogas. Biogas is the gas produced during the decomposition of organic matter in the absence of oxygen. It is a mixture of approximately 45%- 75% methane, 25-55% carbon dioxide and some traces of hydrogen sulphide [7]. This research was focussed on bubbling biogas on four stages with the same molarity thus to improve on more CO2 to be removed. As compared to other research which is one stage bubbling of biogas.
2. Methodology
2.1. Materials and Methods
The biogas gas was collected from Jomo Kenyatta University of Agriculture and Technology in a generation plant that uses cow dung as feed material. A trial experiment was conducted to investigate the suitability of sodium hydroxide solution for the removal of carbon dioxide from biogas and the suitable set up as shown in figure 1. Biogas was passed through the system and samples were collected at the end. The analysis of biogas used before and after the experiment showed a remarkable decrease of CO2 content. The valve at the far end is connected to the last capillary tube and is also meant to collect the gas after the reaction thus provides open/close properties. The other valve hanging is meant to allow biogas into reagent bottles from the source.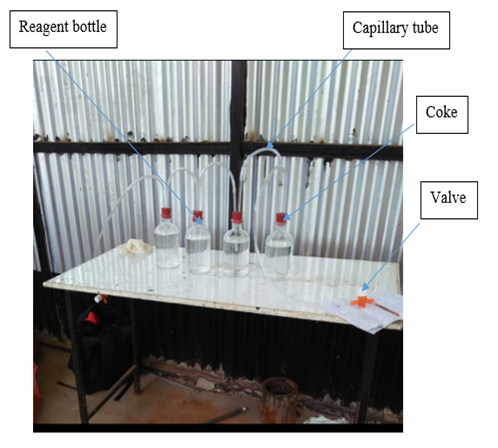 | Figure 1. Trial experiment on carbon dioxide scrubber |
2.2. The Design of the CO2 Scrubber
Design of scrubber was done using inventor software and the design is as shown in figure below. 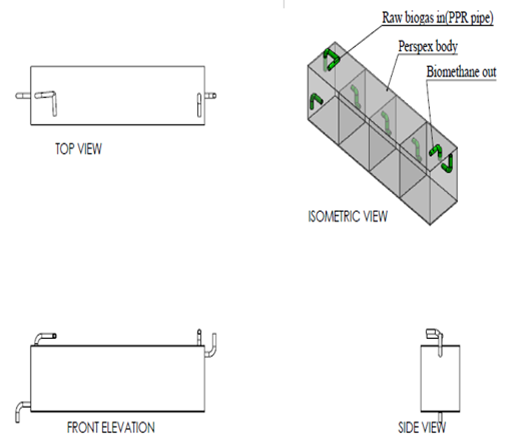 | Figure 2. Experimental design of CO2 scrubber |
2.3. Research Materials Collection
2.3.1. Biogas
The gas was collected from Jomo Kenyatta University of Agriculture and Technology in a generation plant that uses cow dung as feed material. Between the chambers of the scrubber there was no any measuring device to ensure that same amount of the biogas in each chamber moved to next chamber but is assumed because of pressure build up same biogas was scrubbed and biogas being insoluble in the solvent. The composition of the gas was analysed by use of a gas chromatograph in the same university.
2.3.2. Analysis of Biogas
The analyses for methane and carbon dioxide and in the biogas were carried out using gas chromatograph. 1ml of gas sample was taken in syringe and injected into the GC sample port. The injected gas was carried by the mole gas into the column. Inside the column the components were separated by differential partition in between the mobile phase gas and stationary phase liquid. The component that was separated into the gases came out of the column first and was detected by the detector. The one that was separated into liquid phase came out later and was also detected. The readings were displayed in the decoder as a print out in the form of peaks.
2.3.3. Carbon Dioxide Scrubber Horizontal Column
A Perspex sheet was used to make the horizontal column with four compartments meant to carry the sodium hydroxide solution. Biogas was then bubbled through the solution and carbon dioxide was scrubbed. Choice of Perspex is because is non-reactive to sodium hydroxide being a very reactive alkali. Perspex is non corrosive material and transparent thus better choice for this project.
2.3.4. Gas Bags
The gas bags were fabricated from FluoroFilm FEP (fluorinated ethylene propylene) which is the most chemically inert of all bag materials. They are resistant to chemical reaction and also high rigidity and high hardness [8]. These gas bags were bought they were not fabricated by the researcher.
2.3.5. Sodium Hydroxide
An aqueous solution of sodium hydroxide was prepared by adding the solid into a container containing 10 litres of distilled water. The solution was then stirred for well mixing using a stirring rod to produce a homogenous aqueous solution of sodium hydroxide. Four different concentrations were then prepared which were used in scrubbing carbon dioxide from biogas.
2.3.6. Polyvinyl Isolating Valves
These are air operated valves offering normally open/closed capabilities. The valves material was chosen so as to reduce weight on the piping system.
2.3.7. PPR Half Inch Pipes
These polypropylene pipes are very strong, resistant to chemicals and have good welding properties. These were connected to allow biogas flow from the source to the scrubber and then through the chambers lastly to exit where the gas was collected with valves in between to allow open and closed properties.
2.4. Fabrication of the CO2 Scrubber
Perspex was used to construct the scrubber, the sheet was cut and folded to make the required shape. It was 80cm by 18cm by 20cm to give volume of about 28.8litres, the horizontal column was divided into four smaller columns each of volume 7.2litres measuring 20cm by 18cm by 20cm. in all the compartments same concentrations is used in the different chambers to ensure that there is optimum scrubbing of CO2 from biogas. | Figure 3. Carbon dioxide scrubber |
Carbon dioxide scrubber was made in such way that it has four chambers both of them holding the same molarity of sodium hydroxide.
2.5. The Experimental Set Up
The biogas mixture was contacted with the solvent which preferentially absorbs the desired carbon dioxide from biogas stream. The composition of biogas was then measured using gas chromatograph. The gas chromatograph has three main components;• Injection port- this is where the gas is injected and the standard volume is 1ml• Column –where the carrier gas flows and should be constantly flowing• Detection- where the separated components were detectedThe detector used is TCD thermal couple detector during the experiment the column injecting/detecting temperature was 150°C. The column initial temperature was 120°C and column final temperature 120°C.The conditions set during the analysis were;• Speed 10cm/Min• Attenuation/sensitivity 2• Stop time 7minutes• Pressure for helium 120KPa• Flow rate for helium 20ml/MinCarrier gas flows inside and outside of the column, for constant retention time, temperature, flow rate, volume of injection and time of injection should be constant.
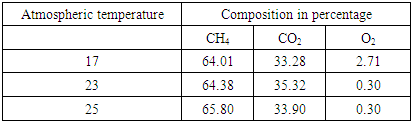
2.6. Initial Composition of Biogas at Different Atmospheric Temperatures
These temperatures are ambient atmospheric temperature measured on different days and different times.Table 1. Initial composition of biogas before scrubbing at different atmospheric temperatures
 |
| |
|
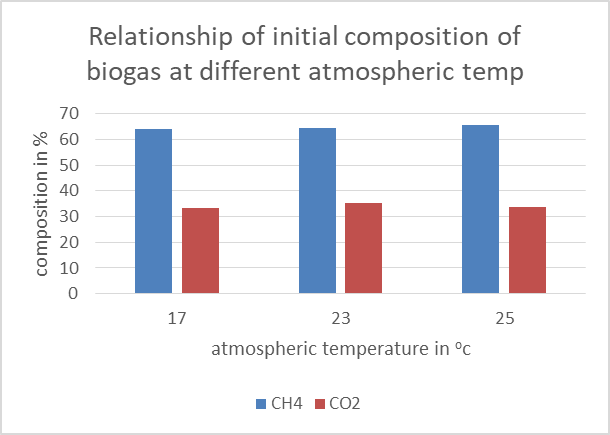 | Figure 4. A graph showing the initial composition of biogas at different atmospheric temperatures |
The graph above shows the different atmospheric temperature the composition of biogas was determined. The recorded composition had no noticeable effect by the atmospheric temperature change.
2.7. CH4 in % in Different Molarities of NaOH
Biogas was passed through different molarities of sodium hydroxide on different occasions and time and percentage composition of the samples were recorded using a gas chromatograph. The table below shows the percentage of methane in composition in different molarities of sodium hydroxide after scrubbing.Table 2. Methane in percentage in different molarities of NaOH
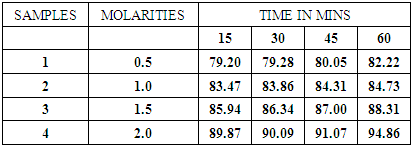 |
| |
|
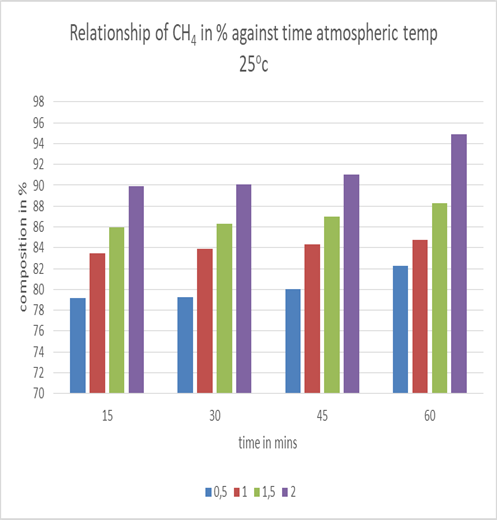 | Figure 5. Effect of different molarities of NaOH on CH4 composition |
From the graph above it shows that as the concentration of NaOH was increased and retention also increased thus percentage composition of methane also increased. This directly was inverse on what happened with carbon dioxide thus it reduced as concentration of NaOH was increased and retention time as well.
2.8. CO2 in % in Different Molarities of NaOH
Table 3. CO2 in percentage in different molarities of NaOH
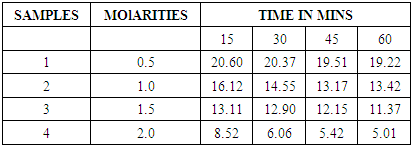 |
| |
|
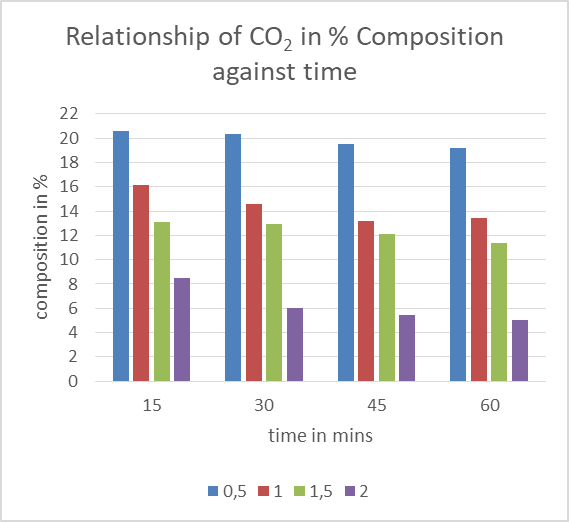 | Figure 6. Effect of different molarities of NaOH on CO2 composition |
The graph above shows the trend CO2 composition after biogas being passed through different molarities of NaOH at different time intervals. As the concentrations of sodium hydroxide was increased as well as time the amount of CO2 in composition percentage reduced subsequently amount of CO2 scrubbed from biogas increased.
2.9. Biogas Compositions in 0.5Molar Sodium Hydroxide
Table 4. CO2 remaining in biogas after reaction in 0.5molar NaOH
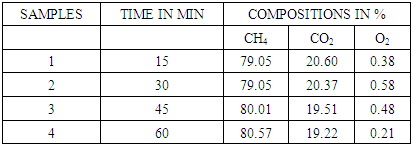 |
| |
|
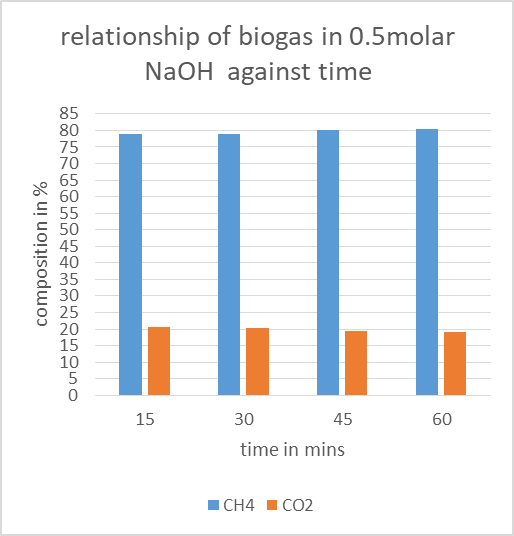 | Figure 7. Effect of 0.5molar NaOH on biogas composition |
2.10. Biogas Compositions in 1Molar Sodium Hydroxide
Table 5. CO2 remaining in biogas after reaction in 1molar NaOH
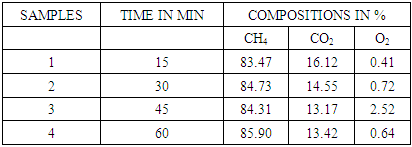 |
| |
|
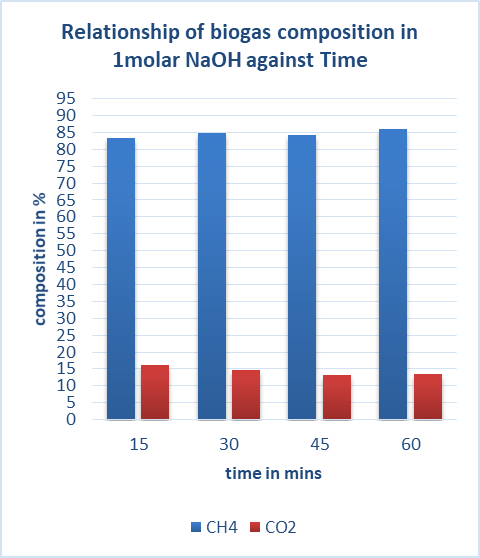 | Figure 8. Effect of 1molar NaOH on biogas composition |
2.11. Biogas Composition after Reaction in 1.5Molar Sodium Hydroxide
Table 6. CO2 remaining in biogas after reaction in 1.5molar NaOH
 |
| |
|
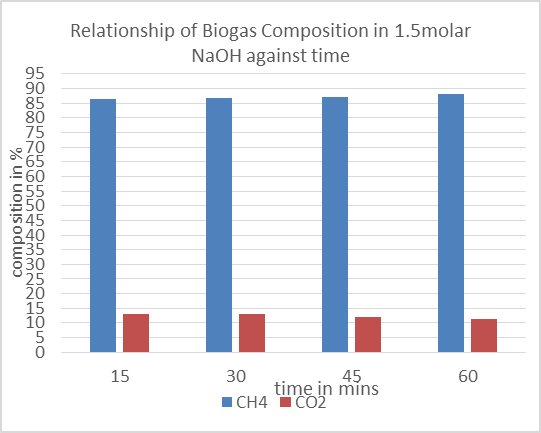 | Figure 9. Effect of 1.5molar NaOH on biogas composition |
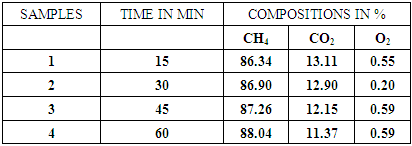
2.12. Biogas Composition after Reaction in 2Molar NaOH
Table 7. CO2 remaining in biogas after reaction in 2molar NaOH
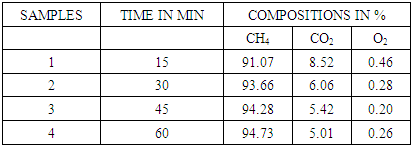 |
| |
|
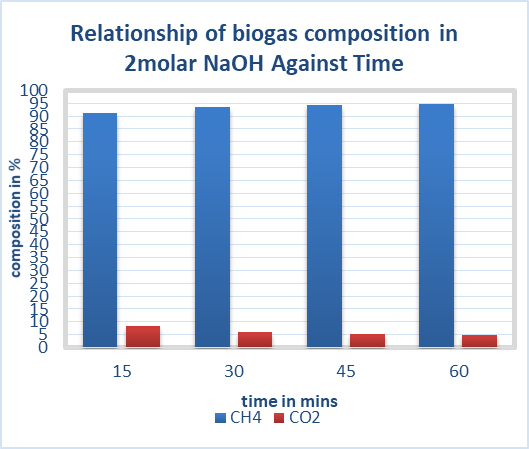 | Figure 10. Effect of 2molar NaOH on biogas composition |
2.13. Discussion
In this experiments, readings of nitrogen were neglected as the gas do not react with sodium hydroxide. The higher the concentration had high absorption capacity irrespective of the time the yield is affected by the concentration.Sodium hydroxide reacted with CO2 according to the following chemical reaction Which can be ionically represented as
Which can be ionically represented as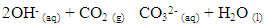 The CO2 removal efficiency for the design was calculated according to the following equation.
The CO2 removal efficiency for the design was calculated according to the following equation. 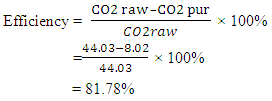 As compared to LPG gas, pure methane has calorific value of around 39.8MJ/kg- 52.3MJ/kg while that of liquefied petroleum gas (LPG) has typical specific calorific value of 46.1MJ/kg. Therefore if biogas is successfully purified can be used for heating with the calorific value similar to that of LPG gas. Contributions of biogas usage include;• Better and cheaper fuel for cooking, lighting and power generation.• Produces good quality enriched manure to improve soil fertility. • Sources are sustainable and can be managed and used indefinitely without degrading the environment.
As compared to LPG gas, pure methane has calorific value of around 39.8MJ/kg- 52.3MJ/kg while that of liquefied petroleum gas (LPG) has typical specific calorific value of 46.1MJ/kg. Therefore if biogas is successfully purified can be used for heating with the calorific value similar to that of LPG gas. Contributions of biogas usage include;• Better and cheaper fuel for cooking, lighting and power generation.• Produces good quality enriched manure to improve soil fertility. • Sources are sustainable and can be managed and used indefinitely without degrading the environment.
3. Conclusions
The carbon dioxide scrubber was successfully designed, fabricated and tested. The concentration of the reagent played an important role in the CO2 removal. When the concentration of absorbent increased, the CO2 removal would be increased to a high efficiency. High removal efficiency was observed at 2M concentration followed by 1.5M, 1M and 0.5M respectively. Results obtained in this work demonstrate that it is possible to achieve the complete removal of CO2 from biogas using NaOH process that operates at ambient temperature. Therefore the usage of chemical absorption is viable method for biogas upgrading in Kenya.
ACKNOWLEDGEMENTS
First and foremost have to give thanks to almighty God for His enduring love and mercy upon me. Secondly, I would like to acknowledge and warmest thanks to my research supervisors: Professor Alex Muumbo and Professor Reuben Marwanga who made this work possible. Their friendly guidance and expert advice have been invaluable throughout all stages of the work. Special thanks goes to my husband for his support and love all throughout my work. Not forgetting the technical staff for the help they rendered to me this was really appreciated.
References
| [1] | International energy agency. (2014). CO2 EMISSIONS from fuel combustion. Executive Director of the IEA. |
| [2] | Soroush Younessi, S. a. (2015). CO2 and its Seques- tration Techniques; Advances in Environmental Biology, 428-432. |
| [3] | Coninck, H. d., & Benson, S. M. (2015). Carbon Dioxide Capture and Storage. Annual Review of Environment and Resources, 7-10. |
| [4] | NIesner, J., Jecha, D., & Stehlik, P. (2013). Biogas Upgrading Technologies: CHEMIICAL ENGIINEERIING TRANSACTIIONS. |
| [5] | Echt, W. (2012). Technologies for efficient purification of natural and synthesis gases. Honeywell UOP. |
| [6] | Tewelde, G. B., Tesfahuney, R. G., Mekonnen, G. A., tewelde, G. B., Tesfahuney, R. G., & Mekonnen, G. D. (2017). Biogas Plant Distribution for Rural Household. Energy and Policy Research. |
| [7] | Shah, D. R., nagarsheth, H. J., and Acharya, P., shah, D. R., Nagarsheth, H. J., & Achaya, P. (2016). Purification of Biogas using Chemical Scrubbing and Application of Purified. Biogas Research Centre, 1-7. |
| [8] | Kauffman, D. J. (2008). Climate change and energy pathways for the Mediterranean. Paris: Alliance for global sustainability book series. |












 Which can be ionically represented as
Which can be ionically represented as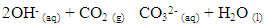 The CO2 removal efficiency for the design was calculated according to the following equation.
The CO2 removal efficiency for the design was calculated according to the following equation. 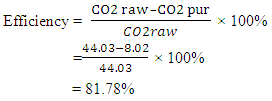 As compared to LPG gas, pure methane has calorific value of around 39.8MJ/kg- 52.3MJ/kg while that of liquefied petroleum gas (LPG) has typical specific calorific value of 46.1MJ/kg. Therefore if biogas is successfully purified can be used for heating with the calorific value similar to that of LPG gas. Contributions of biogas usage include;• Better and cheaper fuel for cooking, lighting and power generation.• Produces good quality enriched manure to improve soil fertility. • Sources are sustainable and can be managed and used indefinitely without degrading the environment.
As compared to LPG gas, pure methane has calorific value of around 39.8MJ/kg- 52.3MJ/kg while that of liquefied petroleum gas (LPG) has typical specific calorific value of 46.1MJ/kg. Therefore if biogas is successfully purified can be used for heating with the calorific value similar to that of LPG gas. Contributions of biogas usage include;• Better and cheaper fuel for cooking, lighting and power generation.• Produces good quality enriched manure to improve soil fertility. • Sources are sustainable and can be managed and used indefinitely without degrading the environment. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





