-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2020; 10(4): 95-101
doi:10.5923/j.ijee.20201004.01
Received: June 18, 2020; Accepted: July 20, 2020; Published: August 15, 2020

Properties of Balanites Aegyptiaca Biodiesel as a Pottential Energy Carrier in the Drylands of Nigeria
Abdulyakin Usman , Ibrahim Ahmad Rufai
Department of Mechanical Engineering, Faculty of Engineering, Bayero University Kano, Nigeria
Correspondence to: Abdulyakin Usman , Department of Mechanical Engineering, Faculty of Engineering, Bayero University Kano, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

There is a huge gap between demand and supply of energy carriers, which needs to be met through increased production of biodiesel using non-edible oil plants, without jeopardizing national food security. The study was conducted to investigate the fuel properties of desert-date (Balanites aegyptiaca)-based biodiesel for possible use as fuel for internal combustion engines. Biodiesel was produced from Balanites aegyptiaca oil using alkali catalyzed trans-esterification method. The fuel properties were determined in accordance with the Association of Official Analytical Chemists (AOAC 1990), American Society for Testing and Material (ASTM D6751) and European standard methods. The average oil yield was 396 g/kg, while the oil extraction efficiency was 88%. Trans-esterification of Balanites aegyptiaca oil revealed high biodiesel yield of 82.7%, 15.9% glycerol with 1.4% stir loss. Analysis of biodiesel fuel properties revealed a kinematic viscosity of 4.7mm2/s at 40°C, higher heating value of 37.5 MJ/kg, acid value of 0.11mgKOH/g, peroxide value of 1.4mEq/kg, iodine number of 68.53 gI2/100g, saponification number of 216 mgKOH/g, density of 886.79 kg/m3 at 15°C and cetane number of 50.42. These results are in accordance with the ASTM and European standard for biodiesel except its higher heating value. The findings from the study suggest Balanites aegyptiaca as a raw material for biodiesel production and its biodiesel as suitable alternative fuel for communities in dryland areas of Nigeria.
Keywords: Dryland areas, Balanites aegyptiaca, Vegetable oil, Trans-esterifcation, Biodiesel, Fuel properties
Cite this paper: Abdulyakin Usman , Ibrahim Ahmad Rufai , Properties of Balanites Aegyptiaca Biodiesel as a Pottential Energy Carrier in the Drylands of Nigeria, International Journal of Energy Engineering, Vol. 10 No. 4, 2020, pp. 95-101. doi: 10.5923/j.ijee.20201004.01.
Article Outline
1. Introduction
- As fossil fuels are depleting, there is need to search for alternative fuel in order to meet the world energy demand. In view of the above, increased attention has been given to biofuels, such as biodiesel, that can be used as an alternative energy carrier in compression–ignition engines. Biofuels have been gaining increased attention as a substitute for petroleum in the transportation sector to mitigate the effects of greenhouse emissions on climate change and offset the depletion of fossil fuels. Oil crops such as palm, sunflower, peanut, soybeans were the largest group of exploitable renewable biomass resources for liquid fuel and energy generation in Nigeria. Chemically, biodiesel consists of mono-alkyl esters of long chain fatty acids derived from vegetable oil and animal fat, designated as B100, and it must meet the special requirements such as the ASTM D6751 and the European standards. The technical and economic advantages of biodiesel are that, it reduces greenhouse gas emissions because it reduces some exhaust emissions; it helps to reduce country’s reliance on crude oil imports and supports agriculture by providing a new labor and market opportunities for domestic crops; it enhances the lubricating property; it is safer to handle, being less toxic, more biodegradable and it is widely accepted by vehicle manufacturers. Feedstock is about 80% biodiesel’s total operating cost, this made it the major economic factor to consider for input costs of biodiesel production. Other important costs are labor, methanol and catalyst, which must be added to the feedstock [1]. Biodiesel is becoming commercialized, but biodiesel obtained from different oil sources have been reported to have different physical characteristics and chemical compositions [2]. Balanites aegyptiaca belongs to the Kingdom: Plantea; Division: Spermatophyta; Subdivision: Angiospermea; Class: Dicotyledonea; order: Balanitales; family: Balanitaceae; Genus: Balanites; Species: aegyptiaca; [3]. Balanites aegyptiaca tree is distributed in West Africa, especially in West African arid and semi-arid regions where the climatic environment and soil are not suitable to produce plants commonly used for the production of biofuels [4]. It is one of the most common but neglected wild plant species of dry land area of Africa and South Asia [5]. It is always green even in the worst drought, with wide ecological distribution [6]. It is sometimes described as a small to medium-sized semi-deciduous tree which attains a height of about 8-10 m and a stem diameter of 30 cm as depicted in figure 1 [7]. The seed (figure 2) has thin brittle epicarp, a fleshy mesocarp and a woody endocarp containing the oil seed or kernel. A mature tree could yield about 10,000 pieces of fruit per year [8]. Balanites aegyptiaca is widely distributed throughout Africa. It is also found in the relatively drier regions of Northern Africa from Mauritania to Nigeria and Ghana, to Egypt, across Palestine, Saudi Arabia and India [9].
 | Figure 1. Balanites aegyptiaca Tree |
 | Figure 2. Balanites aegyptiaca Seeds |
2. Methodology
2.1. Materials
- The materials used in the current study include magnetic stirrer, ground sample of the Balanites aegyptiaca seed kernel. Other materials include, N-hexane, methanol, vegetable oil extracted from the plant’s seed kernel, sodium hydroxide pellets (caustic soda), benzoic acid, ignition thread, B100 (Transesterified Balanites aegyptiaca oil). Soxhlet extractor depicted in figure 3 was the equipment used for the oil extraction. The setup used for biodiesel production is presented in figure 4. Brookfield viscometer was used to determine the kinematic viscosity, while Bomb calorimeter (G Cussons P6310) was used to determine lower heating value of the biodiesel sample. Other equipment used for oil extraction and biodiesel production includes: electronic weighing balance (Mettler Toledo), water bath and magnetic hot plate.
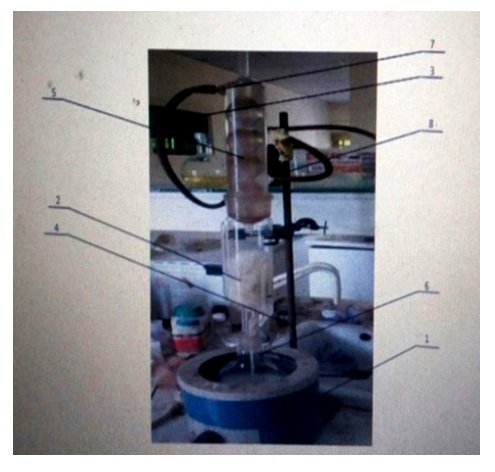 | Figure 3. Soxhlet Extractor. 1: Heating Mantel, 2: Thimble, 3: Hose, 4: Tripod stand, 5: Condenser, 6: Distillation Flask, 7: Siphon Top, 8: Siphon Exit |
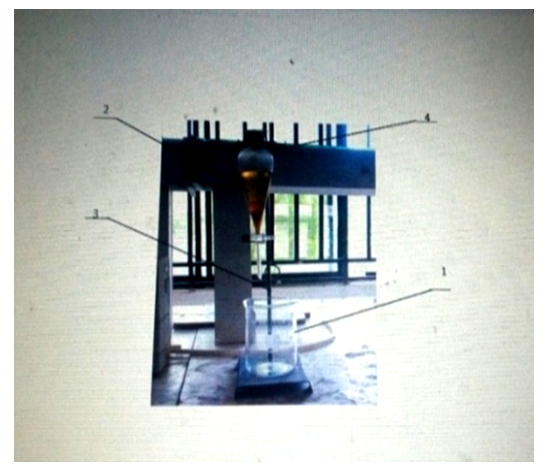 | Figure 4. Biodiesel Separator. 1: Separating funnel, 2: Tripod stand, 3: clamp, 4: Beaker |
2.2. Methods
2.2.1. Oil Extraction Using Soxhlet Extractor
- The aim of this experiment was to extract oil from the sample according to the following procedure. 100g of the seed sample was weighed using electric weighing balance and placed into the thimble of the Soxhlet extraction apparatus. 175ml of n-hexane solvent was poured into the thimble and allowed to extract the oil at 68°C for about 2-4 hours. The extract (mixture of oil and solvent) were then taken to an oven which facilitates evaporating of the solvent and the pure oil was obtained. The procedure was repeated nine times for the remaining 900g seed samples and the readings recorded.
2.2.2. Biodiesel Production Using Alkali Catalyzed Transesterification Method
- The aim of this experiment was to synthesize biodiesel from the oil extracted according to the following procedure. One hundred (100) ml of the oil was measured and poured into a 250 ml conical flask and heated to a temperature of 50°C using a water bath. A solution of sodium methoxide was prepared in a 150ml beaker using 0.5g of NaOH pellet and 30ml of methanol. The sodium methoxide solution produced was then poured gently into the warm oil and stirred vigorously for about 90 minutes using a magnetic stirrer. The mixture was then poured in a separating funnel and allowed to settle for about 24 hours. After settling, the upper layer (biodiesel) was decanted into a separate beaker while the lower layer which consists of glycerol and soap were collected from the bottom of the funnel. The decanted biodiesel was washed with warm water of about 45°C in order to eliminate soluble methanol, excess catalyst and other impurities. The washing process left the biodiesel looking a bit cloudy which indicated the presence of moisture. The biodiesel was heated slowly to a temperature slightly above 105°C in the oven until all moisture present evaporated. The procedure was repeated three times for the remaining oil samples and the readings recorded.
2.2.3. Analysis of Fuel Properties
- The fuel properties of the biodiesel sample such as iodine value (IV), saponification value (SV), peroxide value (PV), acid value (AV) and density at 15°C were determined according to Association of Official Analytical Chemists (AOAC) official method. The kinematic viscosity was determined at 40°C using a Brookfield Viscometer as shown in figure 5 according to the American Society for Testing and Materials (ASTM D445), while the higher heating value was determined according to ASTM D7042 using Bomb calorimeter as shown in figure 6, while the cetane number was obtained empirically according to [24].
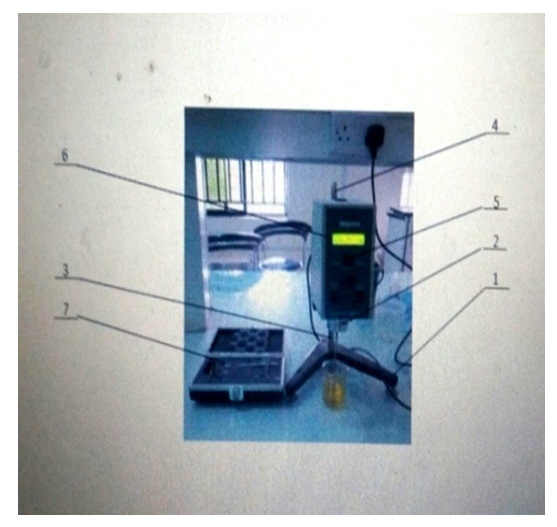 | Figure 5. Brookfield Viscometer. 1: Guard Legs, 2: Spindle, 3: Extension Shaft, 4: Clutch level, 5: Switch, 6: Screen, 7: Extension Shaft Box |
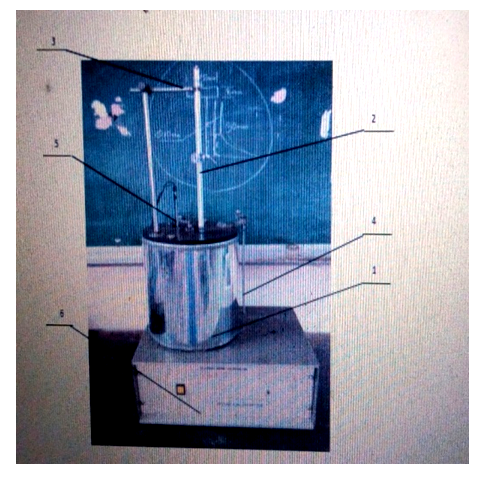 | Figure 6. Bomb Calorimeter. 1: Calorimeter Vessel, 2: Beckman Thermometer, 3: Thermometer Clamp, 4: Stirrer Drive, 5: Charged bomb, 6: Firing Terminals |
3. Results and Discussion
3.1. Oil Extracted and Biodiesel Produced from Balanites aegyptiaca
- Balanites aegyptiaca seed kernel (1.0 kg) obtained from about 12 kg of its fruits collected yield an average of 396 g (426 ml) oil using soxhlet extractor. The extraction efficiency indicates that the extractor is capable of extracting about 88% of the available oil as presented in Table 1.
|
|
3.2. Fuel Properties of Balanites aegyptiaca Biodiesel
- The results in Table 3 show average fuel properties of Balanites aegyptiaca biodiesel. The acid value is an important factor in evaluating the quality of biodiesel as it measures the amount of acids present in the fuel (hydro peroxide in biodiesel that further oxidized in to acids). The Balanites aegyptiaca biodiesel acid value was determined to be 0.11 mgKOH/g as presented in Table 3. The result showed appreciable consistency with the ASTM and EN standards. Similar results have also been reported by [29] for Peanut (0.3 mgKOH/g), Palm kernel (0.26 mgKOH/g) and Moringa biodiesel (0.18 mgKOH/g). The small variations in acid value could be due to the variation in composition, Production procedures and conditions.
|
4. Conclusions
- The industrial utilization of Balanites aegyptiaca oil for the production of biodiesel is favorable because of its oil yield capacity from the plant and its limitation for utilization as edible oil. The promising plant having oil yield of 396 g/kg (42%) with 88% extraction efficiency revealed high oil yield, and fact that the soxhlet extractor could extract about 88% of the available oil present in the seed. The oil having biodiesel yield of about 82.7% shows the effectiveness of alkali catalyzed transesterification method in synthesizing biodiesel from the plant oil, its prospect as better alternative raw material for biodiesel production, and promise for local farmers in dryland areas.The Balanites aegyptiaca biodiesel having kinematic viscosity of 4.7 mm2/s, calorific value of 37.5 MJ/kg, acid value of 0.11 mgKOH/g, peroxide value of 1.4mEq/kg, iodine number of 68.53 gI2/100g, saponification number of 216 mgKOH/g, density of 886.79 kg/m3 and cetane number of 50.42 possessed biodiesel fuel properties as the fuel properties are proper in respect of ASTM D 6751 and EN 14214 specification standard, except its higher heating value. The study revealed that the biodiesel shows good fuel properties. Thereby, Balanites aegyptiaca is a potential feedstock for the production of biodiesel which can be used as an alternative energy carrier in the dryland areas of Nigeria. Development of the technology for harnessing the potentials in Balanites aegyptiaca will certainly contribute to the energy security of this vulnerable region. In addition, a cradle-to-grave analysis of the use of Balanites aegyptiaca biodiesel, as an energy carrier, is necessary to ensure its environmental, social and economic sustainability.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML