-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2019; 9(2): 25-35
doi:10.5923/j.ijee.20190902.01

Evaluation of Process Parameters for Biodiesel Production from Vegetable and Palm Waste Frying Oils Using a Homogeneous Catalyst
Aworanti O. A. , Ajani A. O. , Agarry S. E. , Babatunde K. A. , Akinwumi O. D.
Biochemical Engineering and Biotechnology Laboratory, Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
Correspondence to: Ajani A. O. , Biochemical Engineering and Biotechnology Laboratory, Department of Chemical Engineering, Ladoke Akintola University of Technology, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Biodiesel from waste frying oil is an effective alternative fuel to conventional diesel and can be directly used as fuel in a diesel engine without any modifications to the engine. The major objectives of this work were to produce and compare the biodiesel yield from waste frying vegetable oil (WFVO) and waste frying palm oil (WFPO) using transesterification process. The physicochemical characterization of the biodiesel as well as the effects of process variables on biodiesel yield were evaluated. Also, process conditions for optimum production of biodiesel were determined. The WFVO and WFPO with methanol and catalyst were heated in a hot plate-magnetic stirrer at a temperature of 60°C and operated at 300 rpm. Potassium hydroxide (KOH) was used as catalyst. One-Factor-at-A-Time method was used to select the optimum levels of process variable that gives high biodiesel yield. The acid value, free fatty acid, density, kinematic viscosity, pour point and saponification value were (5 and 28.5 mgKOH/g), (2.5 and 14.25%), (946 and 908 kg/m3), (0.0385-0.0469 Pa-s), (310 and 223°C) and (181.2 and 175.87 mgKOH/g), respectively. From the results of the analysis, it shows that the physiochemical characteristics of WFVO and WFPO were within the standard value of EN14214 and ASTMD-6751. From the results, the optimum process variables for transesterification process using KOH catalyst were found to be; reaction time of 90 minutes, methanol to oil molar ratio of 12:1 and catalyst loading of 1.5 wt%. At these optimum conditions, the optimum yield of biodiesel obtained from WFVO and WFPO were found to be 97% and 90%, respectively. In addition the acid value, free fatty acid, density, kinematic viscosity, iodine value, refractive index, flash and pour point of biodiesel produced from WFVO and WFPO were (0.26 and 0.57 mgKOH/g), (0.13 and 0.29%) (863 and 864 kg/m3), (0.0041 and 0.0060 Pa-s), (2.210 and 1.435 gI2/100g), (1.44 and 1.45), (-3 and -5°C), (164 and 129°C), respectively. Thus, the results of the analysis falls within the specification range of international standards for biodiesel specifications. Thus, in comparism, the transesterification of WFVO resulted in higher biodiesel yield than WFPO. Conclusively, both WFVO and WFPO have potential to be used for bio-diesel production.
Keywords: Biodiesel, Waste frying oils, Transesterification, Homogenous catalyst Potassium hydroxide, Methanol
Cite this paper: Aworanti O. A. , Ajani A. O. , Agarry S. E. , Babatunde K. A. , Akinwumi O. D. , Evaluation of Process Parameters for Biodiesel Production from Vegetable and Palm Waste Frying Oils Using a Homogeneous Catalyst, International Journal of Energy Engineering, Vol. 9 No. 2, 2019, pp. 25-35. doi: 10.5923/j.ijee.20190902.01.
Article Outline
1. Introduction
- The demand for energy is increasing every day due to increase in population growth, industrialization and price of petroleum. Availability of conventional energies will be limited in the near future, due to oil depletion [1]. Production process of fossil fuel has led to global climate change, environmental degradation, human health problems and emission of greenhouse gases, As a result of these associated problems, there is a need to search for an alternative energy such as renewable energy for the world [2]. The renewable energy that can be used to substitute petroleum-derived fuels are biofuel (Biogas and Biodiesel), solar energy, and producer gas, hydrothermal and geothermal [3-4]. Biodiesel has been considered as one of alternative fuels as it is a clean renewable fuel, a carbon monoxide emission reducer, non-toxic, bio degradable, and environmental friendly fuel [5-6]. Biodiesel has a good fuel properties such as lower emission of carbon dioxide, high flash point, high cetane number and good lubrication and it has almost the same characteristics with conventional diesel fuel characteristics (physical and chemical characteristics) therefore it can be used without mixing with petroleum derived diesel [7-9]. Biodiesel can be produced from edible oil such as groundnut oil, palm oil, coconut oil, soybean oil, sunflower oil, castor oil and rapeseed oil and non-edible oil like waste cooking or frying oil as well as from oil extracted from waste crops and animal fat by transesterification process [10]. Transesterification is a reaction which occur between lipid and alcohol in the presence of catalyst to form esters (biodiesel) and by product (glycerol) at a particular temperature and reaction time. It can also be defined as the process which involves the reaction alkyl-alcohol with long chain esters in the presence of a catalyst to give mono-alkylesthers and glycerol [11-13]. The quality and quantity of biodiesel fuels depends on the types of raw materials (edible oil, non-edible oil and fat), types of chemical (catalyst and methanol), operating condition, free fatty acid and alcohol to oil ratio [6,14]. Types of catalyst use for transesterification reaction in biodiesel production are homogeneous and heterogeneous catalysts. The homogeneous catalysts are classified into two, acid and base catalysts, while the heterogeneous are classified into three, acid, base and enzymatic catalysts. The rate of reaction for base catalyzed transesterification is higher and produce high amount of biodiesel at reduced reaction time than that catalyzed with acidic catalyst, while enzyme catalyst is a good catalyst for transesterification due to it moderate temperature and high yield but it is expensive. Therefore, the preferred process for biodiesel production is homogeneous alkaline transesterification because the catalyst is faster and cheaper than other catalysts [7,15,16]. Sodium hydroxide and potassium hydroxide are the most used homogenous alkaline catalyst, these catalysts have high conversion at mild conditions and reduced reaction time and the advantages of homogeneous catalysts over heterogeneous catalysts are less corrosive, quantity of alcohol required is small, and does not require sophisticated reactors [17]. It is reported that alkali catalyst is sensitive to free fatty acid in feedstock used for biodiesel production. Thus, oil such as pure vegetable oil with less than 1 wt% free fatty acid is required as the feedstocks. However, if the oil selected for the production of biodiesel contain high free fatty acid, an esterification process must be applied as a pre-treatment in order to eliminate or reduce the free fatty acid before proceeding to the transesterification process [18-22]. It is observed that the cost of biodiesel production using pure refined oil as feedstock is more expensive than petroleum derived diesel and this is due to the high cost of the refined oil used as feedstocks [23,24,10]. The use of waste frying oil as feedstock to replace refined vegetable oil in biodiesel production is an alternative way to reduce the feedstock cost and also using the waste oil will solve the problem of waste disposal in the environmental [25-27]. Waste frying oil is defined as used frying oil obtained from frying process. During the frying process, the triglyceride in the refined vegetable oil break down to form diglycerides, monoglycerides and free fatty acid (FFAs) and these constitutes the composition of the waste frying oil [28]. The compound formed during this frying process increases the molecular mass of the oil but reduces the volatility of the waste oil [29,6]. The properties of the waste frying oil are different from the refined oil, this is due to the mass transfer that occurs between the material being fried and the oil as well as the chemical reactions that occurs during the frying process which include oxidation, hydrolysis, and polymerization [28]. Waste frying oil has a higher proportion of saturated fatty acids and renewability of better oxidation stability [30,31,6]. According to the ASTM standard, the density, viscosity, acid value, and free fatty acid of waste frying vegetable oil should range between 896-950 kg/m3, 29-40 cst, 1.5-2 mg of KOH/g of oil and 0.1-0.25% respectively [32,6], while for waste cooking palm oil, the density, viscosity, acid value, and free fatty acid of waste cooking vegetable oil range between 912-916 kg/m3, 133.33-137.53 mm2/s, 0.2-0.824 mg of KOH/g of oil and 0.1-0.412% respectively [33,34]. Many research work have been done on how to reduce the high cost of production and increase the quantity and quality of biodiesel fuel from waste frying vegetable oil and waste frying palm oil using homogeneous catalyst [35-38,28,34]. However they have not worked toward determination of possible optimum level of process variables for statistical optimization. The aim of this paper is to investigate the potential use of WFVO and WFPO as feedstock for biodiesel production. The effects of catalyst loading, methanol to oil ratio and reaction time on biodiesel yield were evaluated. Also process conditions for optimum production of biodiesel were determined.
2. Materials and Methods
2.1. Sample Collection and Feedstock Preparation
- Waste frying vegetable oil obtained from refined palm olein (WFVO) and waste frying palm oil obtained from mesocarp (reddish pulp)(WFPO) were collected from various restaurants in Ogbomosho, Oyo state, Nigeria. Methanol, sulphuric acid, propanol, potassium oxide and phenolphthalein were obtained from a chemical store in Ibadan, Oyo state, Nigeria. A round bottom -flask was used as a reactor and magnetic stirrer (with hot plate) was used as a stirring and heating medium. The waste frying oil (vegetable oil and palm oil) were heated at 70°C, thereafter the hot waste frying oils were filtered using filter paper in order to remove impurities, suspended particles and inorganic materials present in the waste oils. The waste oils were filtered to avoid impairment of oil quality causing reduction in the productivity of the transesterification reaction and also to avoid generation of undesirable by-products that will hurt the final product [39].
2.2. Characterization of the Waste Frying Oil and Biodiesel Produced
- The characterization of the waste frying oils (WFOs) and biodiesel produced were done according to the ASTM standards. Properties analyzed were density, viscosity, acid value, iodine index, saponification value, Refractive index, free fatty acid (%), flash point and pour point.
2.2.1. Determination of Density of Waste Frying Oils and Biodiesel Produced
- The density measurement was carried out according to ASTM standards D-5 [40]. Density of waste frying oils and biodiesel produced at 15°C were determined by gravimetric analysis, 25 ml waste frying oils was measured with a glass cylinder and the mass of the oil was determined using an electronic scale. The density was calculated using Equation 1. [40]
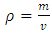 | (1) |
2.2.2. Determination of Viscosity of Waste Frying Oils and Biodiesel
- The viscosity measurement was carried out according to ASTMD- 445 [40]. The falling-ball viscometer was used to measure the viscosity of liquid by measuring the time required for a ball to fall under gravity through a sample-filled tube that is inclined at an angle. The measurement was carried out in triplicate and the average viscosity was estimated. The viscosity and kinematic viscosity can be determined by Equation 2 and 3 respectively. [40]
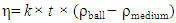 | (2) |
 | (3) |
2.2.3. Determination of Refractive Index of Waste Frying Oils and Biodiesel
- In this experiments the Abbe refractometer (VEE GEE Model C10, Thomas Scientific, USA) was used to measure the refractive index. The light ray pitched on the interphase of phases then it was refracted. The impact angles, rebound and the refraction were measured between a ray running perpendicular to the phase interface. Ray break is a result of differences in the speed of light in both phases. Refractive index is the ratio of the speed of light in phases, the light passes through. Its principle is the detection of limit angle fracture (βmax), which is the maximum possible angle fracture where the angle of impact is close to 90°. [40]
2.2.4. Determination of Saponification Value of Waste Frying Oil and Biodiesel
- The saponification value was determined according to ASTM standards D-5558 [43,42,41]. The saponification value was obtained by washing 2 grams of oil into excess alkaline solution of potassium hydroxide, then the mixture sample was boiled for 45 minutes for complete saponification, 1ml of phenolphthalein indicator was added. The solution was titrated with 0.5N hydrochloric acid (HCl) until the pink colour just disappeared. A blank determination was conducted simultaneously with the sample. The saponification valve was calculated based on Equation 4: [44-45]
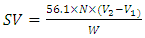 | (4) |
2.2.5. Determination of Acid Value of Waste Frying Oil and Biodiesel
- Acid value was determined according to ASTM-D 1980 [41]. The acid value was determined by titration of the sample (oil or biodiesel) dissolved in the mixture of ethanol-toluene with a standardized titration solution of KOH. In this method, a weighed amount of the sample (oil or biodiesel) was added into a flask and it was dissolved in 50 ml of ethanol-toluene mixture (1:1 v/v); phenolphthalein was added as an indicator. KOH was used as titrant solution. The titration process was stopped when the solution turned into pink color. The acid value (AV) was calculated using Equation 5: [10]
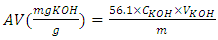 | (5) |
2.2.6. Determination of Iodine Number of Waste Frying Oils and Biodiesel
- This was determined according to the ASTM standard-5554 [1]. The iodine value was obtained by weighing 0.5 g waste frying oil and pour into Erlenmeyer flask. Chloroform solution (10 ml) and 25 ml of Hanus solution (Iodine-Bromide Reagent) were added, shaken until all oil were well blended and then kept in a dark room (to prevent light reaction) for 30 minutes. 10 ml of 15% KI solution was added. Titration was done with a solution of 0.1 N Na2 S2 O3 and the indicator used was 1% starch. The titration was stopped when a clear solution was obtained. The iodine number was calculated according to Equation (6) [44]
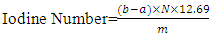 | (6) |
2.2.7. Determination of Free Fatty Acid of Waste Frying Oils
- The free fatty acid of waste frying oil was done according to the procedure reported by [46]. The free fatty acid was determined by titration of the sample (oil) dissolved in the propanol solutions, phenolphthalein was used as an indicator with a standardized titration solution of KOH. In this method, 5 g of oil was dissolved in 25 ml of propanol then 5 drops of phenolphthalein indicator was added to the oil-propanol solution, thereafter the oil-propanol solution with phenolphthalein indicator was titrated with 0.1N KOH solution until the colour of the solution turn to pink. The free fatty acid (FFA) was calculated using Equation 7:
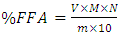 | (7) |
2.2.8. Determination of Pour and Flash Points of Biodiesel
- The pour and flash points were determined according to the ASTM standard D97, D25100-8 and D56, respectively.
2.2.9. Determination of Methanol to Oil Ratio
- The molecular weight of the oil was determined using Equation 8 [18]:
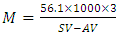 | (8) |
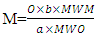 | (9) |
2.3. Experimental Procedure
2.3.1. Desacidification of Waste Frying Oils by Esterification Reaction
- The esterification reaction was done according to the procedure reported by [10]. 100 ml of waste frying oils were measure and heated to 60°C, then sulphuric acid (0.14 ml) and methanol (55 ml) were added to the waste frying oil, the mixture was pour into a round- bottom flasks and then stirred with magnetic stirrer at 800 rpm for 60 mins. Thereafter, the mixture was allowed to settle in a separation funnel for 2 hrs in order to achieve 2 distinct liquid phases (water at the top and preheated oil at the bottom).
2.3.2. Transesterification of Treated Waste Frying Oils
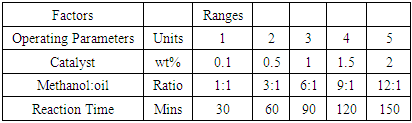
- The transesterification reaction for the production of biodiesel from treated waste frying oils were carried out in accordance with the procedure of Aworanti et al., [7]. One-factor -at-a time (OFAT) approach was used to evaluate the possible optimum level of the operating parameters that can be used for the production of maximum biodiesel. The ranges of the operating parameters used are stated in Table 1. Different amount of methanol and catalyst as shown in Table 1 were weighed and mixed vigorously with magnetic stirrer in order for the catalyst to be dissolved and form potassium methoxide solution. Constant volume of pretreated waste frying oil (100 ml) in a round bottom flask was heated to 60°C, then the potassium methoxide solution was poured gently into the heated waste frying oils. The entire mixture was stirred with hot plate magnetic stirrer at 300 rpm and the temperature was maintained at 60°C. After the process, the mixture was poured into a separating funnel and kept for 24 hours so as to separate the glycerin from the biodiesel. The separation segment are glycerol layer at the bottom and biodiesel layer at the top. Thereafter, the physicochemical properties of biodiesel derived from the transesterification of WFVO and WFPO were determined and compared with EN 14214. The biodiesel yield was calculated using Equation 10
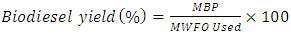 | (10) |
|
3. Results and Discussion
3.1. Characterization of Wastes Frying Oils Used in Biodiesel Production
3.1.1. Fatty Acid Number
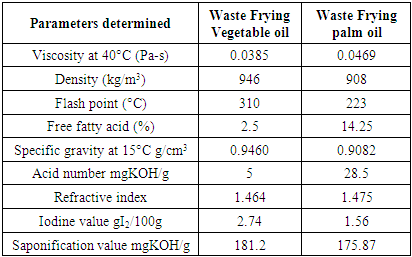
- The result of the properties of waste frying oils analyzed in this study is presented in Table 2, it was observed that the free fatty acid values of the various samples were more than 2%, which justifies pretreatment (esterification) of the waste frying oil samples in order to reduce the free fatty acids in the wastes oil [47]. This high percentage of free fatty acids (FFAs) in the WFVO and WFPO is due to the effect of frying on the properties of WFVO and WFPO, such as temperature and frying time. When the vegetable oil is subjected to thermal stress during frying, its chemical and physical characteristics completely change. The quantity of heat to fry and quantity of water in the frying oil increases the hydrolysis of triglycerides, therefore it leads to high percentage of free fatty acids (FFAs) in the WFVO and WFPO [48].
|
3.1.2. Saponification Number
- The saponification number of waste frying vegetable oil and palm oil used for this research work were 181.2 mgKOH/g and 175.9 mgKOH/g, respectively. The saponification number indicates the amount of potassium hydroxide (KOH) needed to saponify (convert to soap) one gram of oil. It was reported in the literature that saponification number for waste frying vegetable oil ranges from 176 to 187 mg KOH / g oil, while for waste frying palm oil ranges from 177 to 200 mg KOH / g [33]. [Sánchez et al. 51] reported that the saponification number of waste cooking oil was 196.98 mg KOH / g oil. The saponification number of waste frying vegetable oil and palm oil used in this work falls within the literature report. Increase in the saponification number of WFO compare to refined oil are caused by oxidation and polymerization reactions during frying [52].
3.1.3. Density Value
- The density of waste frying vegetable oil and palm oil used for the transesterification reaction are 946 kg/m3 and 908kg/m3, respectively. The results obtained are within the range reported in the literature (0.9625 g / ml and 0.9772 g / ml) [53].
3.1.4. Viscosity Value
- The kinematic viscosity value of the waste frying vegetable oil and palm oil are 0.0385 Pa-s and 0.0469 Pa-s, respectively. [López et al. 33] reported the viscosity value of waste cooking oil to be 0.04Pa-s, which is in the range with the viscosity value of waste frying oil used in this research. Density, specific gravity and kinematic viscosities have been described as the most basic and important fuel properties or parameters because fuel performance indicators such as cetane number and heating values are correlated with these parameters [54-56]. Increase in the viscosity number of WFO compare to refined oil are caused by oxidation and polymerization reactions during frying [52].
3.1.5. Flash Point and Pour Point
- Flash point is defined the temperature at which biodiesel burns when in contact with ignition source. Flash points of the waste frying vegetable oil and waste palm oil were 310°C and 223°C respectively, the acid value of the waste frying vegetable oil and palm oil for this work were 5mgKOH/g and 28.5 mgKOH/g, respectively.
3.1.6. Acid Value
- The acid value is one of the most important properties used to determine biodiesel quality and the percentage of free fatty acids contained in each oil [57]. It shows the amount of corrosive acid as well as oxidation products present in the oil. The acid value is the number of milligrams of KOH required to neutralize all acid in 1g of sample. From literature reviewed the acid value should be lower than 0.50 mgKOH/g specified by ASTM standard [58]. According to [Meher et al. 59] the acid value of waste cooking oil for transesterification reaction should be in the range of 0.2 to 0.824 mgKOH/g. Comparing this result with the literature, it shows that the oil used in this research work has a very high acid value and it is inferred that it has been over-used. Therefore the oil must be pretreated in order to reduce free fatty acids.
3.1.7. Iodine Value
- The iodine value of waste frying vegetable oil and palm oil were 2.74 and 1.56, respectively. The iodine value indicates the amount of this compound which can absorb the vegetable oil and palm oil in unsaturated bonds, that is, the larger the index value the greater adsorption on the double bonds present in the oil [60]. From literature the iodine value of waste cooking oil ranges between 60 – 70 mg/100 g [33], the iodine values obtained in this work were very low which indicate low adsorption rate on the double bonds and also the compound that absorb the vegetable oil and palm oil in unsaturated bonds is low.
3.1.8. Refractive Index
- The refractive index of waste frying vegetable oil and palm oil used for this research work were 1.464 and 1.475, respectively. The refractive index of a medium is a measure of how much the velocity of a wave is reduced inside that medium.
3.2. Properties of Biodiesel Produced
3.2.1. Fatty Acid Number
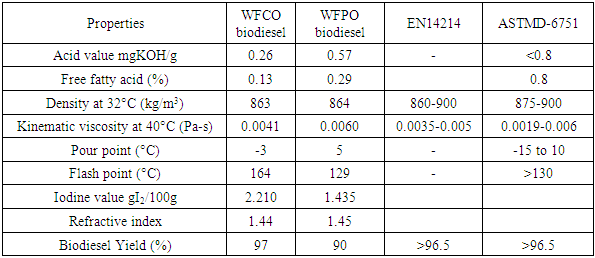
- The result of free fatty acid of biodiesel analyzed in this study is presented in Table 3. The free fatty acid of biodiesel production from WFVO and WFPO are 0.13 mg/KOH and 0.29 mg/KOH respectively. According to the data of ASTMD-6751, the maximum value of free fatty acid in biodiesel is 0.8 mg/KOH. The result shows that the biodiesel product has free fatty acid value that is in accordance with the standard. The acid value is one of the most important properties for biodiesel quality check. High acid value can cause sediment in the fuel system and corrosion of the media. The higher the acid value the lower the quality of biodiesel [1,61].
3.2.2. Iodine Value
- The result of iodine number of biodiesel analyzed in this study is presented in Table 3. The iodine number of methyl ester from WFVO and WFPO are 2.210/100 g and1.4352/100 g respectively. The results obtained meets the SNI standard [62,1]. If the iodine value of biodiesel produced is higher than the standard of 115 g/100 g it will lead to polymerization and formation of deposits in injector’s nozzle and piston rings at the start of combustion.
3.2.3. Density
- The result of density of biodiesel analyzed in this study is presented in Table 3. The density of transesterification process from WFVO and WFPO are 860 kg/m3 and 900 kg/m3 respectively. The results obtained meets the EN14214 and ASTMD-6751 standard. Density provides information on how the fuel will work in diesel engines. High density value indicate some impurities in the biodiesel [61].
3.2.4. Viscosity
- The result of viscosity of biodiesel analyzed in this study is presented in Table 3. The viscosity value of biodiesel from WFVO and WFPO are 0.0041 Pa -s and 0.0060 Pa-s. The results of the analysis falls within the specification range of the ASTM D-6751 standard. Viscosity is defined as fluid resistance to the flow rate of a mm-sized capillary. If the viscosity value is high it will lead to formation of oxidized polymeric compounds and this can lead to the formation of gums and sediments that clog the filters in the engine [63].
3.2.5. Acid Value
- The result of acid value of biodiesel analyzed in this study is presented in Table 3. Based on results obtained, the acid number from biodiesel produced from WFVO and WFPO falls within the range of the ASTMD-6751 standard. Acids can be formed when traces of water is presence in the biodiesel which result into hydrolysis of the esters to form alcohol and acids [63]. The acid number increases with an increase in peroxides because the esters first oxidize to form peroxides which then undergo further reactions, including a split into more reactive aldehydes which further oxidize into acids. Acid number indicates the level of free fatty acids (FFAs) present in biodiesel. Acid value lower than 0.5 mg KOH/g is ideal as fuel for vehicle. A high acid value can have a strong solvency effect on rubber seals and hoses in the engine, thereby causing premature failure. It may also leave deposits, which can clog the fuel filter or drop fuel pressure.
|
3.2.6. Flash Point and Pour Point
- The result of flash point and pour point WFVO and WFPO of biodiesel analyzed in this study is presented in Table 3. Flash point is the temperature at which biodiesel burns when in contact with ignition source. The values of flash point of the biodiesel produced from waste frying vegetable oil and waste frying palm oil were 164°C and 129°C. These values fall within the range of biodiesel flash point standard (ASTM D6751). Pour point has been described as an important parameter for low temperature operation of a fuel also the lowest temperature at which fuel can flow. It is the temperature at which wax becomes visible when the fuel is cooled and it is sufficient to gel the fuel [35]. The value of pour point of the biodiesel produced from waste frying vegetable oil and waste frying palm oil were -3°C and 5°C. These values fall within the range of biodiesel pour point standard (ASTM D6751). Lastly the appearances of the biodiesel produced was noticed, it was observed that the biodiesel produced from waste frying vegetable oil and waste frying palm oil are of different colour. Their colours are yellow and brown respectively.
3.3. Effect of Operating Variables on Biodiesel Yield
3.3.1. Effect of Catalyst Loading on Biodiesel Yield
- The biodiesel yields obtained from the transesterification of WFVO and WFPO at different amount of catalyst (KOH), using 9:1 methanol to oil ratio, operated at stirring rate of 300 rpm and temperature of 60°C for 90 min are shown in Figure 1. It is observed from the plot that biodiesel yield obtained from WFVO and WFPO increases with increase in catalyst loading up to 1.5 wt%. It could be seen that the yield respectively increases to 86% and 90% for WFPO and WFVO when the catalyst loading increased to 1.5 wt% at 90 min, while the yield decreases to 55% and 80% for WFPO and WFVO as the catalyst loading increased above 1.5 wt%. Thus catalyst loading that resulted in optimum biodiesel yield of 86% and 90% was found to be 1.5wt% for both WFPO and WFVO, respectively. This observation may be due to the fact that, an increase in catalyst amount up to 1.5 wt% increases the total number of active sites, resulting in an increase in biodiesel conversion [64,65], while an increase in catalyst loading above 1.5 wt% makes the reactant and catalyst mixture too viscous leading to problems with mixing and poor diffusion of the reactants, thus resulting in a decrease in the biodiesel yield [32,64,66,67]. Furthermore, decrease in yield may also be attributed to the fact that the solubility of methanol in oil is low and increasing catalyst loading provides more active sites to adsorb the products consequently, the yield of biodiesel decreases [68]. Also the low biodiesel yield at catalyst loading above 1.5 wt% may be due to the attainment of mass transfer limitation (rate determining step) between the reactant and catalyst [69].
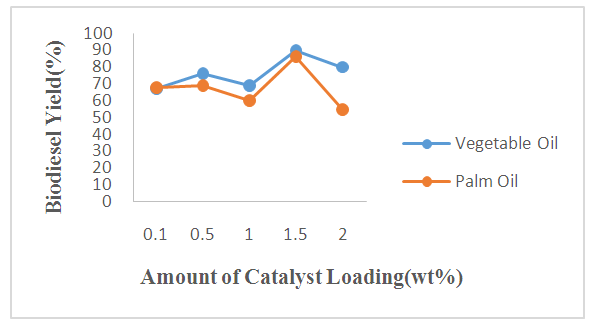 | Figure 1. Effect of Amount of Catalyst Added on the Biodiesel Yield from WFVO and WFPO |
3.3.2. Effect of Methanol/Oil Molar Ratio on Biodiesel Yield
- The yields of biodiesel obtained from transesterification of WFVO and WFPO by using different values of molar ratio of oil to methanol are shown in Figure 2. Thus, the optimum methanol to oil ratio was determined by carrying out the transesterification reaction with various methanol to oil ratios (1 : 1, 3 : 1, 6 : 1, 9:1 and 12 : 1) using a catalyst loading of 1.5% and a time of 90 min at temperature 60°C. It was observed in the plot that, at methanol to oil ratio of 1:1 and 3:1, a high biodiesel yields from both WFPO and WFVO was never achieved, an increase in relation to molar proportion ranging between 6:1 to 12:1 led to a substantial increase in biodiesel yields obtained from WPVO and WFVO from 60% to 90% and 75% to 97% respectively was noticed in the plot. Thus optimum methanol to oil ratio that resulted in optimum biodiesel yields of 90% and 97% was found to be (12: 1) for both WFPO and WFVO, respectively. Whereas the biodiesel yields obtained from WFPO and WFVO were decreased at methanol to oil ratio of 1:1. The methanol to oil ratio is another important factor which affects the biodiesel yields. In order to increase the biodiesel yields and to keep the equilibrium on the right side of the reaction, it is necessary to increase the methanol in the reaction [12]. Hypothetically, every mole of biodiesel is a result of one mole of methanol and 1/3 of a triglyceride mole from the transesterification response. Stoichiometrically, 3 mol of methanol is required to produce one mol of glyceride [70].
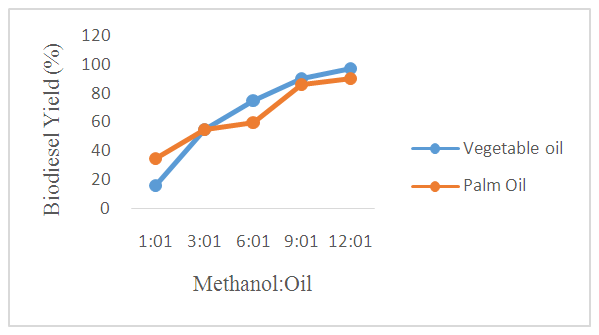 | Figure 2. Effect of Methanol:Oil on Biodiesel Yield From WFVO and WFPO |
3.3.3. Effect of Reaction Time on Biodiesel Yield
- The influence and effect of reaction time on biodiesel yields was examined under the following operating conditions: 1.5 wt%, temperature of 60°C and molar ratio of 12:1 and the biodiesel yields obtained at different time intervals are shown in Figure 3. The experimental result shows that the biodiesel yields obtained from WFPO and WFVO increases with increase in time up to 90 min, It could be seen that the yields increase to 90% and 97% for WFPO and WFVO when the time increased to 90 min, while the yields decrease to 70% for both WFPO and WFVO respectively as the time increased above 90min. Thus the time that resulted in optimum biodiesel yields of 90% and 97% was found to be 90 min for both WFPO and WFVO, respectively. The yields deteriorated after 90 min because hydrolysis of esters may start to occur with a further increase in the reaction time, which results in more fatty acids forming soap [71]. Additionally, a back reaction may take place after reaching the equilibrium since the reaction is reversible, subsequently decreasing the yield [30,72-74,67, 75].
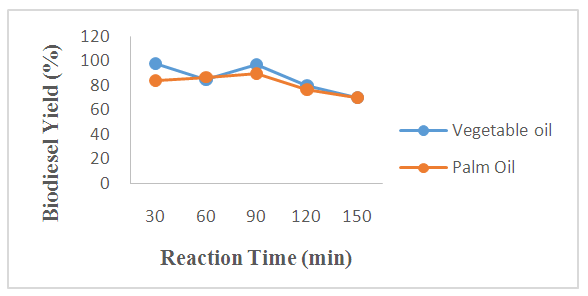 | Figure 3. Effect of Reaction Time on the Process of Transesterification using WFVO and WFPO |
3.4. Assessment of Optimum Biodiesel Yield from WFVO and WFPO
- Assessment of biodiesel yields obtained from the transesterification of WFVO and WFPO carried out at optimum conditions of 1.5 wt% catalysts, 1:12 oil to methanol molar ratio, 90 min reaction time and at a temperature 60°C is shown in Figure 4. The experimental results reveals that transesterification of WFVO at the above optimum conditions resulted in the optimum yield of 97% when compared with the transesterification of WFPO which produced an optimum yield of 90% at the same optimum operating conditions. The reason for this observation may be due to the high value of free fatty acid present in the waste frying palm oil. A Similar observation has been reported by [Ali et al. 35, Hossain et al. 12] who respectively obtained a biodiesel yield of 68.9% from wastes soybean oil using 0.5% KOH as catalyst 94.4% from wastes cooking oil using 0.4 wt. % KOH.
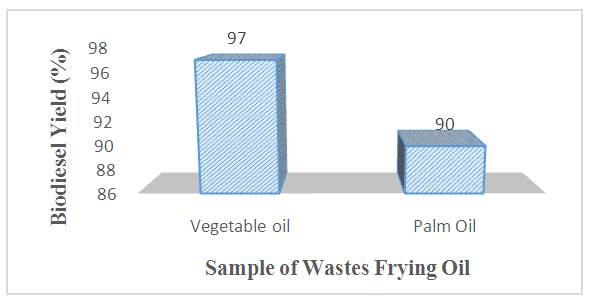 | Figure 4. Biodiesel Yield from different wastes frying oil |
4. Conclusions
- The optimum conditions for the produced biodiesel were: methanol to the oil (12:1), amount of catalyst loading (1.5 wt %), and reaction time (150 min). The maximum yield of biodiesel was 97% obtained from transesterification process of waste frying vegetable oil. It was found out that that important fuel properties biodiesel produced at optimum condition met the biodiesel ASTM standard. For carrying transesterification, batch reactors are preferred over continuous because of easy assemblage, maintenance, inexpensive and easy to design. Obtaining higher yield of product depends upon the quality of oil used. The parameters affecting the reaction were identified to be methanol to oil molar ratio, the catalyst used and its amount, reaction time and the reaction temperature.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML