-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2018; 8(3): 67-88
doi:10.5923/j.ijee.20180803.03

Impact Assessment of Metal-Based Octane Boosters: A Literature Review
Benjamin Afotey
Department of Chemical Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Correspondence to: Benjamin Afotey, Department of Chemical Engineering, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Metal-based octane boosting gasoline additives such as methyl cyclopentadienyl manganese tricarbonyl (MMT) and Ferrocene (dicyclopentadienyl iron) have been used for many years. Their usage, especially in modern gasoline vehicles equipped with advanced emissions control systems, has been controversial. Concerns have been raised that combustion products from MMT and Ferrocene containing fuels adversely affect engine components and emissions control systems performance and durability, as well as result in health and environmental impacts. In contrast, other researchers have reported that combustion products from MMT and Ferrocene fuels do not cause harm and have no measurable effects on regulated emissions. This study provides comprehensive review of the literature regarding the impact assessment of metal-based octane boosters. It details the impact of MMT and Ferrocene on vehicular engine components, health and environment, octane boosting effectiveness, claimed benefits, current gasoline specification limits, current market penetration, legislation, cost level indication and stakeholder’s position. Several test programs have been conducted with the use of metal-based octane boosters on a wide range of vehicle model years, technology types and test conditions. Reports by automakers over this body of literature concluded that these octane boosters have detrimental effects on vehicle engine components such as catalyst plugging, spark plugs misfire and oxygen sensor malfunction. Further reports concluded that emissions of metallic oxides on combustion of gasoline containing these octane boosters result in health and environmental effects. Additional credible reports also documented extensive regulatory programs consideration and implementation to control the limits of these octane boosters in gasoline and help reduce the metallic emissions that impact human health. Stakeholders, therefore, remain extremely concerned about organometallic additives’ in markets around the world and hence the Worldwide Fuel Charter recommends against their use in gasoline applications.
Keywords: Metal-based octane boosters, Impact assessment, Vehicular engine components, Health and Environment
Cite this paper: Benjamin Afotey, Impact Assessment of Metal-Based Octane Boosters: A Literature Review, International Journal of Energy Engineering, Vol. 8 No. 3, 2018, pp. 67-88. doi: 10.5923/j.ijee.20180803.03.
Article Outline
1. Executive Summary
- The ban of tetraethyl lead in the 1970s led to an immediate search for a substitute that will enhance octane and have positive impact on modern gasoline vehicles equipped with advanced emissions control systems as well as public health and welfare. The search soon ended with Ethyl Corporation discovering MMT, a manganese-based octane booster, as the alternative to tetraethyl lead. The combustion of MMT releases manganese compounds into the air that are associated with neurological disorders similar to Parkinson’s disease [1-4]. Akin to tetraethyl lead, this additive raises concerns about public health risk of raising ambient concentrations of heavy metals and disabling effect these metals may have on advanced emission control devices on vehicles. Automakers and scientists began encouraging actions against MMT that is similar to the action being taken on lead, but Afton Chemical Corporation, which succeeded the Ethyl Corporation in 2004, defends its product as safe and effective. Up to date, MMT is heavily marketed in developing countries as a convenient and low-cost replacement for lead, but not widely used in developed nations [1].Manganese (Mn) forms part of a balanced diet in food that helps in the production of enzymes for processing blood sugars into energy. Inhalation, however, is potentially a more dangerous route of exposure than ingestion [2, 3, 5, 6]. When orally taken, manganese passes through the digestive system, where the liver is able to regulate the concentration entering the bloodstream. When it is inhaled into the lungs, however, it bypasses the liver and enters the bloodstream directly, where it travels unregulated to the brain and potentially accumulates to toxic levels [1].Occupational studies have shown that manganese causes neurotoxic effects and others relating to the pulmonary and reproductive system. The progressive neurological damage it produces in workers is called Manganism and to date there is no known successful treatment or cure for it [3, 5, 4]. The brain is the most vulnerable organ in the body to high concentrations of manganese. Manganese compounds can cross the blood-brain barrier and accumulate in regions responsible for motor control, cognition, emotions and learning. The manganese compounds emitted from the exhaust pipe includes highly soluble manganese sulfates, which mix into the bloodstream more rapidly and may be more hazardous than other forms of airborne manganese. In countries where MMT was used, traffic density correlated with higher manganese concentrations. Manganese was also higher in urban areas than in rural areas where traffic is lower. It is unlikely that widespread MMT use would produce concentration of manganese as high as those seen in occupational studies, but a group of epidemiological studies have shown that even low levels of airborne manganese can increase the incidence of Parkinson-like disorders [1, 6].Certain population groups are exposed to varying concentrations of manganese in certain microenvironments and that may lead to different health risks. An extensive study conducted on environmental effects of Mn exposure on adult population in Brescia, Italy reveals that the odds ratio for a physician-diagnosed Parkinsonian Disturbances (PD) was 1.034 per 10 ng/m3 increase in Mn in total suspended particles. Therefore, the researchers concluded that exposure to ambient Mn advances the age of PD diagnosis, thus strengthening the hypothesis that exposure to Mn adds to the natural neurons attributable to the ageing process. Additional work on the children population showed that an impairment of olfactory function and motor coordination in different age groups like children might be caused by Mn due to transport of Mn through the olfactory tract leading to dopaminergic dysregulation. Additional behavioral testing results in adolescents (age 11-14) showed that those pupils had a significant impairment of motor coordination, hand dexterity and odor identification, which was associated with soil Mn. Further tests result on the effects of Mn in drinking water on children showed that Mn intake by tap water was positively correlated with the impairment of school children at the age of 6-13 years. For example, a 10-fold increase of Mn in water was associated with a decrease of 2.4 IQ points (p<0.01), with a median Mn concentration in tap water of 34 µg/L (range: 1-2700 µg/L). For infant’s population, a study regarding the relationship between maternal and umbilical cord blood Mn levels and birth weight in a cohort of 470 mother-infant pairs showed that maternal blood Mn levels during pregnancy were associated with birth weight in full-term infants in a non-linear pattern. In addition, research results indicate that, pre and post natal overexposure to Mn to the fetus or newborn may have crucial consequences for the developing child with potential harmfulness for the fetus and hence the age of 12 months is a sensitive developmental window specific to Pb-Mn interaction. For post-occupational population, results suggested that past exposure to Mn might have lasting consequences on neuropsychiatric symptoms as those workers showed higher scales for anxiety, hostility and depression compared to controls. These findings locate the focus of Mn intoxication on other neurological effects than injury of neurons, but towards psychological effects and emphasize the danger of Mn still after a long time of acute exposure. Finally, it is known nowadays that neurotoxicity holds a time variable consistent of two parameters; the exposure duration as well as the period of life when it occurs [7].Since the 1990s, there has been a growing concern against the use of MMT and other metal-based gasoline additives by the health community and recent developments are renewing calls for action. Stakeholders including the World Health Organization, the United States government, the Canadian government and the European Union have adopted risk-based standards for airborne manganese compounds, recognizing that without regulation this pollutant poses a threat to public health. In addition, Mn compounds have been listed as hazardous air pollutants by the U.S EPA. Concerned organizations such as the American Journal of Industrial Medicine in a published paper called for an immediate ban of manganese addition to gasoline in all nations. The American Academy of Pediatrics which played an important role in the policy debate to phase out leaded gasoline strongly disapproves the addition of MMT to the US gasoline, and recommended that metallic additives such as MMT, ferrocene and others must be regulated by government or be phased out [1].Several recent studies on MMT use in gasoline show more certainty about vehicle impacts. Automakers such as Volkswagen have long complained that the presence of MMT in gasoline has a negative effect on the durability and functionality of emissions relevant components. Apart from plugging of catalysts due to manganese oxides, experience with MMT in fuel can cause other adverse effects such as spark plug misfire or oxygen sensor biasing/malfunction, manganese containing deposits in the exhaust system, deposits in piston ring grooves and wear on piston rings and ring grooves. Ford reported catalyst failures experienced by Ford gasoline vehicles in China. It was reported that, on the dates that the failures occurred, China had significant amount of manganese in the market, about 12.2 mg Mn/L in 2007 and 11.2 mg Mn/L in 2008 [8].A study of the impacts of MMT on low emissions vehicles in 2002, which used high density cells in catalytic devices, found that MMT increased hydrocarbon (HC) emissions over 100000 miles and caused seven of eight vehicles to exceed emissions certification standards. A published paper by Ford Motors in 2004 compared vehicles used in the 2002 study and found that the increase in vehicle exhaust emissions were due to the reddish-brown deposits on the cylinder head, spark plugs and the catalyst. Other studies conducted, however, by the metal-based octane booster producing companies, such as Ethyl, also reported the positive effects of MMT in fuel on tailpipe emissions, which include a decrease in the amount of carbon monoxide (CO), a more substantial reduction in nitrogen oxides (NOx), no evidence of an increase or decrease in unburnt hydrocarbons. The Ethyl testing in this case was performed on newer engines with lower vehicle mileage.Further, researchers from German car manufacturers, Porsche performed a study of the impact of MMT on emissions and performance of the 2004 model-year Porsche Carrera, a vehicle with a horizontally opposed six-cylinder engine certified to Euro 4 emission standards. The engines were operated on Super-Plus (EN 228) and Super-Plus with MMT (15 mg Mn/L). In comparison with the additive-free version, the engine using Super Plus with MMT yielded 5% loss of engine power and 3% decrease of maximum torque, 6% higher exhaust-gas back pressure at nominal power and up to 5% higher specific fuel consumption, HC emissions in the EU test increased by 54%, exceeding the Euro 4 limit by 11% (increase of HC emissions by 30%), NOx emissions in the EU test increased by 14% and CO emissions remained similar on both fuels. In Canada, given the fundamentally different conclusions reached by the auto industry and Afton, the Canadian government considered conducting an independent or “third party” review of the effects of MMT. This review became moot, as a result of the voluntary phase-out of MMT use by Canadian refiners from 2003 to 2005. However, data collected in anticipation of the review and while MMT was still in use in Canada, clearly demonstrate the adverse impacts of MMT on advanced technology vehicles. These data demonstrated that MMT in Canadian gasoline resulted in severe catalyst plugging to at least 25 models of 1999 to 2003 model-year vehicles produced by nine manufacturers, and which accounted for approximately 85% of Canadian light-duty vehicle sales in 2006. Also, after MMT use was voluntarily halted by refiners, data showed that catalyst plugging cases in Canada quickly diminished [8, 9].Considering the various studies of MMT and its impact on vehicles emissions control systems and public health with each interest group finding support for their respective positions on the issues and in the absence of independent confirmation, given the preponderance of emission impacts highlighted by the vehicle industry, it can be concluded that these studies provide enough reason to put an immediate halt to the use of MMT. Restricted use of MMT in the developed countries and many developing countries is due to increasing number of voluntary and regulatory bans on MMT. Since 1976, a ban has been in place in the U.S. State of California. In Brazil, Germany and in the Czech Republic, laws and regulations also ban the use of MMT.The European Council and Parliament placed limits on manganese stricter than in the U.S., and eventually as strict as New Zealand standards. A national law in the U.S. bans MMT use in reformulated gasoline, which constitutes 39% of the fuel supply. A separate regulatory limit restricts manganese to a maximum of 8.3 mg/L in the remainder of the nation’s fuel, and as of 2007 a voluntary ban by fuel suppliers further restricted MMT to less than 1% of the supply. Other countries and regions including India, Canada and the European Union have similar bans or limitations in place. MMT is being used in China to date, but strict government controls currently (after 2014) in place in Beijing limit the concentration to 2 mg Mn/L which is similar to the regulatory limit in New Zealand whilst in South Africa, the most of fuel sold does not contain MMT. This trend indicates that major steps taken to restrict MMT use are increasing in number in both developed and developing nations. Refinery investments alone can improve the octane number of fuel without the need for metallic additives and hence policy makers should be encouraged to embrace it [1].The precautionary principle can assist policy makers seeking guidance regarding the use of MMT and other metallic additives since it is aimed at taking the appropriate and responsible steps to prevent a potential health treat in the search of cleaner, cost effective and environmentally friendlier way of fuel production. Policy-makers are hence encouraged to take action to prevent the use of MMT while health research continues and significant uncertainties persist, to pursue alternative means to boost octane to replace the burden of proof on Afton Chemical to respond to scientific uncertainties over the safety of its products, and to call for independent verification of the evidence.With the knowledge about metal additives’ impact, it would be unwise to heed any argument to the contrary, especially from manufacturers and suppliers with a conflict of interest. A more credible and reliable arbiter for the safety of this product is the medical community. The lesson learnt from tetraethyl lead use and recommendations from public health researchers caution us against the use of MMT and other metallic additives.
2. Introduction
- Methyl cyclopentadienyl manganese tricarbonyl, MMT [CH3C5H4Mn(CO)3] and Ferrocene [Fe(C5H5)2] are metal-based octane boosters used to improve the octane number of gasoline. However, their uses have been controversial for many years, and have gained significant attention following the phase-out of tetraethyl lead in gasoline. While they may have a lesser impact on the proper operation of modern emission control systems than lead poisoning, experience shows that significant harmful effects are associated with the use of these octane boosters over time [10].Experience with metal-based additives worldwide suggests that manganese and Iron deposits on engine and emission control components results in spark plug misfire, oxygen sensor malfunction and catalytic converter plugging. Of even greater concern is the impact of metallic fuel additives on the new and emerging technologies, which are designed to satisfy more stringent future environmental legislation being introduced worldwide. To meet new laws, motor vehicle manufacturers are forced to use high cell density catalysts. Such catalysts are mounted closer to the engine and they are exposed to higher temperatures. Such designs are more sensitive to the fuel. Additionally, when using fuels containing metallic additives, data shows that catalysts and other components within the emission control system are poisoned by deposits that also block the channels in the catalyst [10].Further, health scientists are opposed to the use of metallic fuel additives, especially MMT. Combustion of MMT releases manganese, a potent neurotoxin when inhaled. Therefore, health scientists urge policy makers at all levels of government to adopt a position that is in the interest of public health and welfare [8, 10].
3. Background
- History of Methyl cyclopentadienyl manganese tricarbonyl (MMT) UseMMT was first marketed by Ethyl Corporation in 1959, as a supplement or replacement for tetraethyl lead (TEL). It has been used in both leaded and unleaded gasoline, but did not find widespread use in the U.S until the period of mandated lead phasedown, which began in 1974.The MMT-based anti-knock package marketed by Ethyl is known as HITEC 3000TM (and other similar product names). By 1976, MMT was used throughout the U.S. and was being present in approximately 49% of all gasoline, at a typical concentration of 12 mg Mn/gal [9].The use of MMT in gasoline has been controversial over the past few decades. Several publications provide good chronologies of MMT’s use in the U.S and Canada gasoline [1, 11-15]. A brief summary of this chronology is presented as follows:In 1976, due to concerns regarding potential adverse effects on vehicle emissions and emissions control systems, the California Air Resource Board (CARB) issued a ban on the use of manganese-based additives in all gasoline. A waiver of this rule would be required to allow the use of MMT in California. To date, no such waiver has been requested, and the ban of MMT in California gasoline remained in effect.The 1977 amendments to the U.S Clean Air Act (CAA) permitted manganese additives to be used only in leaded gasoline, although this Act also gave the EPA administrator authority to waive the ban is the additive was shown to not cause or contribute to an exceedance of vehicle emissions standards. In 1978, Ethyl Corporation applied for a waiver for the use of MMT in gasoline at concentrations of 8 and 16 mg Mn/L. (8 mg Mn/L is normally equivalent to 30.3 mg Mn/gal). EPA denied the waiver application on the grounds that Ethyl failed to demonstrate that vehicle emissions or emissions control devices would not be harmed [9].In 1981, Ethyl Corporation submitted another waiver application to permit use of MMT at concentrations equivalent to 4 mg Mn/L (15.15 mg Mn/gal). This application was also denied on the basis that Ethyl had failed to demonstrate that no emissions harm would result.In 1990, additional CAA amendments were passed by Congress, conditionally banning the use of manganese-based additives in all reformulated gasoline (as opposed to conventional gasoline). A waiver would be granted if manganese is shown not to increase pollutant emissions through the completion of a waiver test program. [Reformulated gasoline is required in those regions that experience the highest ozone concentrations, in the U.S. (including all of California), as well as other areas that have voluntarily “opted-in” to the federal reformulated gasoline (RFG) program].In 1990-1992, Ethyl submitted a new waiver application to permit use of MMT in gasoline at levels up to 8 mg Mn/L. Based upon the extensive set of emissions data presented by Ethyl, EPA concluded that MMT at this level would not cause or contribute to a failure of emissions control devices in use at that time. Nevertheless, the waiver request was denied due to concerns regarding potential health risks as airborne manganese. Ethyl appealed this decision on the grounds that EPA did not have the authority to make a ruling based on public health. In 1995, the U.S Court of Appeals agreed with Ethyl’s position, and ordered EPA to grant a waiver authorizing the use of MMT in conventional gasoline (not reformulated gasoline) at concentrations up to 8 mg Mn/L [9].This waiver is still in effect today. However, since the late 1990s’, major refineries operating in the U.S have voluntarily eliminated MMT from all their gasoline. Since that time, actual manganese levels in U.S gasoline have been very low, although levels up to 8mg Mn/L are still permitted by law, in non-reformulated gasoline.The history of MMT usage in Canadian gasoline is different from the U.S. With the phase-out of leaded gasoline in the late 1970s, MMT found widespread usage in Canada. Although there was no legal limit on MMT concentrations, the Canadian General Standards Board (CGSB) established a voluntary standard of 18 mg Mn/L in 1978. The national mean concentration in Canadian gasoline in 1993 was 9 mg Mn/L [9].In 1997, due to concerns that MMT use could compromise the effectiveness of vehicles’ on-board diagnostic (OBD) and emissions control systems, Canada passed the Manganese-Based Fuel Additives Act. This act prohibited the importation of MMT into Canada, and the trade of MMT between provinces. After legal challenge, pertaining to restrictions on commerce, this Act was rescinded, thus reverting to the CGSB voluntarily MMT limit of 18 mg Mn/L in Canadian gasoline. However, just prior to implementation of Canadian-specific Tier 2 exhaust standards in 2004, all major Canadian refiners voluntarily eliminated MMT from their gasoline supply. Since that time, actual manganese levels in Canada gasoline had been very low. MMT use of up to 18 mg MN/L continued from the mid-1970s through the 2003 to 2005 phase-out period, and since 2005, however, MMT had not been used in Canada [8, 9]. Several other countries had also established regulations regarding MMT in gasoline. A listing of these regulations (as of 2008) was provided in a report by the International Council on Clean Transportation (ICCT) and the European Automobile Manufacturers Association (ACEA) [9].History of Ferrocene UseFerrocene is a dark orange colored powder, freely soluble in hydrocarbons. It is available from the Associated Octel as the additive PLUTOcen. To date, ferrocene additive had struggled to gain industry acceptance. The basic problem appears to be the erosive nature of the combustion products. There is increasing interest in ferrocene; however, the depth of analysis is much less than that for MMT. Further, there are several alternative suppliers of ferrocene and quality standards of the additive may be a concern [16].
4. Impact on Engine: before and after Treatment
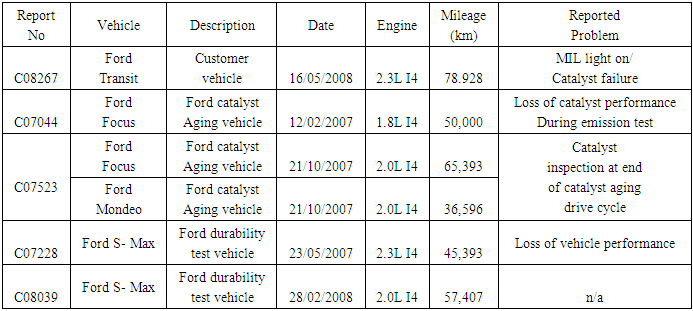
- A number of research works had been conducted to determine the impact of MMT on vehicular engine. One of the world’s automakers, Volkswagen reported that the presence of MMT had a negative effect on the durability and functionality of emissions relevant components. These findings were based on experience of experiments on test benches but also from the operation of vehicles in the field, from countries around the world where MMT is used in gasoline, e.g. South Africa and China as well as parts of Eastern Europe, Asia and Argentina [8].Plugging of catalysts due to manganese oxides is observed when MMT is added to the fuel as seen from catalysts operated in the Chinese market. Figure 1 shows deposits of manganese oxides on a catalytic converter operated in a vehicle from the Chinese market.
 | Figure 1. Deposits of manganese oxides on a catalytic converter operated in a vehicle from the Chinese market (front surface and detail) [8] |
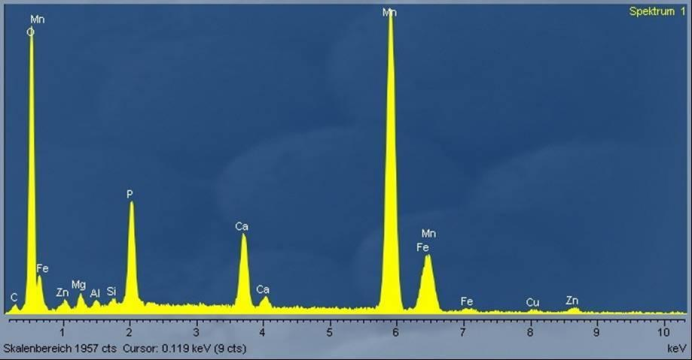 | Figure 2. Spectrum of plugging material on a catalytic converter [8] |
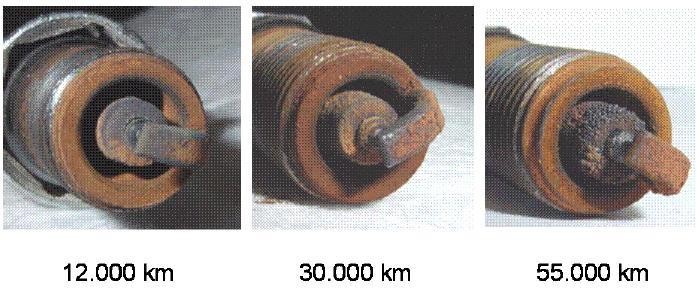 | Figure 3(a). Deposits of manganese oxides on spark plugs [8] |
 | Figure 3(b). Deposits of manganese oxides on oxygen sensors [8] |
|
 | Figure 4. Catalyst frontal area: 90% blocked (manganese content of deposit: 75%) [8] |
5. Impact on Health and Environment
- Hoekman S. Kent reported that the impact that results from exposure of manganese (Mn) through inhalation is quite different from the impact through ingestion. Mn is somewhat unusual with respect to toxicity in that it is relatively non-toxic to humans, except for its effect on the brain. High exposure to airborne Mn can lead to neuro-degenerative disease known as manganism, which has symptoms similar to Parkinson’s disease [2, 3, 4, 9]. Further, according to the classification provided by companies to European Chemical Agency (ECHA) in REACH registrations, manganese is fatal in contact with skin, fatal if inhaled, toxic if swallowed, and is very toxic to aquatic life with long lasting effects. Further classification provided by companies to ECHA in CLP notifications identifies that this substance causes damage to organs through prolonged or repeated exposure and is suspected of causing cancer [24].Biologically, manganese is considered as an essential metal important to mitochondrial oxidative processes for all living mammals, but may also be toxic at high concentrations. Manganese does not occur as a free metal but exist in eleven oxidative states, with only the manganese (II) and manganese (III) oxidative states being of biological significance. Both deficiency and excess of manganese have been associated with detrimental health effects. Excessive intake of manganese either through inhalation or ingestion may result in pathology, particularly to the central nervous system. Excessive exposure via inhalation had been shown to cause effects on the lungs and accumulate in the brain, causing irreversible brain disease, to some extent similar to Parkinson’s disease. These effects are well documented in occupational settings, where correlations between low doses of exposure, blood manganese levels, and neurological health outcomes had also been reported. The authors noted that an estimated 500,000 to 1.5 million people in the United States have Parkinson’s disease, and physicians should consider manganese exposure in its differential diagnosis [2, 25].Additionally, Kent and other researchers reported [5, 9, 25, 26] that combustion of gasoline containing MMT produces emissions of manganese oxides, including MnO, MnO2, and Mn3O4, manganese phosphate and manganese sulfates [27, 28]. These emissions are in the form of fine particulate matter, which are typically red or brown in color. The particles were originally determined as having approximate size of 0.1-0.4µm diameter. More recent work has shown that significant fraction of the particles emitted at the tailpipe were larger than 0.5µm but almost all were in the respirable fraction, < 5µm. while Mn3O4 was initially believed to be the dominant form of Mn particulate emissions, more recent work has shown that the manganese sulfate and phosphate are major contributions, consisting of 70-90% of the respirable manganese emitted [9, 27, 29, 30].Further, he reported that from health perspective, personal exposure levels are more important than ambient concentrations. Several published studies had discussed human exposures to Sydney, and elsewhere. Most studies showed very low exposure levels, typically in the range of 0.01-0.02µg/m3, though considerably higher levels occurred in some situations. There was little consensus regarding the potential health impacts of MMT usage in gasoline-even among regulatory agencies. For example, a 1996 paper by Health Canada researchers concluded: “thus, exposure to respirable Mn is considered low for 98-99% of the population, and the contribution from the combustion of MMT in gasoline is not likely to represent a substantial health threat to Canadians”. At about the same time, a paper by EPA researchers concluded: “given the information that is available at present and the uncertainties discussed here, a reasonable basis exists for concern regarding potential public health risks, especially for susceptible subpopulations, if MMT were to be used widely in unleaded gasoline” [9].Other researchers reported that “manganese is a nutrient that is necessary for the proper function of the human body. It helps to produce enzymes like hexokinase, superoxide dismutase and xanthine oxidase for processing blood sugar into energy and for preventing diseases like cancer and renal failure”. However, it also falls into a category of neurotoxic heavy metals like cadmium, lead and mercury. The body must have manganese to function properly, but must regulate concentration carefully. When it absorbs manganese in food, it has developed a way of taking only as much as it needs. When food makes its way from the digestive system to the circulatory system, it passes through the liver, which is well adapted to filter out high levels of manganese. It does this so well that instances of poisoning are rare in healthy individuals, although children may still be at risk. Individuals who are sick with liver diseases or malnutrition cannot perform this function properly, so excessively high amounts of manganese in food and water can poison them [8].It is further reported by ACEA that “the human body, however, is less able to protect itself against manganese that travels through the air. When it enters the body, it penetrates deep into the lungs where it transfers into the bloodstream, bypassing the liver and making its way unfiltered to the brain. When airborne manganese passes through the nose, neurological pathways can transport it directly to the brain. These mechanisms explain why airborne manganese is much more dangerous than food borne. Manganese is safe only when filtered through a healthy digestive system, not when inhaled through the air” [8].Research work by Michalke and Fernsebner was consistent with the work of other researchers on manganese (Mn) being an essential nutrient in diverse ways. According to them, Mn is important in biochemical reactions of several enzymes including Mn-dependent superoxide dismutase, which place an important role in iron metabolism and is required for proper brain function. They also reported that, in contrast to its physiological attributes, elevated levels of Mn can result in toxic neurological effects, presumably caused through the mechanism of oxidative stress, whereby inhalation is the primary route of concern for occupational health effects. These neurotoxic effects cause a series of symptoms, such as adynamia/fatigability, sialorrhea, cephalalgia, sleep disturbances, muscular pain and hypertonia, masklike face, gait changes, reduced coordination, hallucinations, and mental irritability, finally leading to Mn-induced Parkinson-like disease, called manganism [7, 11].Their work also discussed Mn exposure scenarios in the last century, having changed from the acute, high-level exposure conditions, responsible for the occurrence of manganism, to chronic low-level exposure of MN. On one hand, this change may be due to improved workplace protection for workers with potentially high Mn exposure, such as welders, smelters, workers in battery factories etc., resulting in less cases of acute manganism. On the other hand, there is an increased, chronic Mn exposure to parts of population living close to industrial vicinities with emission of Mn containing dust or living close to high-frequented traffic routes with Mn containing car exhausts from MMT charged fuel [7, 31].The work further addressed the impact of manganese on population in their epidemiological studies. According to them, manganism had always been linked with Mn intoxication of miners, industrial workers or welders, who had occupationally been susceptible to high concentrations of Mn dust during their working life. However, given the recent situation with progressive industrialized emission worldwide, the use of MN as fungicides (Maneb, Mancozeb) or as fuel additive (MMT) in some countries has resulted in the environmental sources of Mn increasing. As a consequence, the problem of Mn neurotoxicity has become a great public health concern due to diverse factors for several members of population such as adults, children, infants and post-occupational workers [7].For the adult population, an extensive study was conducted from four different ferro alloy industries, which were operating until 2001 in the province of Brescia, Italy regarding the effects of environmental Mn exposure on population. The results of the study showed that Mn concentrations in settled dust of each municipality were significantly higher in the surroundings and downwind from the industrial factories. The result also showed that an environmental exposure to Mn is associated with an increased prevalence of Parkinsonian Disturbances (PD). This prevalence of Parkinsonian disturbances by exposure to Mn might also be linked to genetic factors. Therefore, the group developed a concept of susceptibility to classify individuals as prevalent for PD. Therefore, mutation of genes were discussed which played important pathogenic roles in both Parkinsonism and in the regulation of Mn transport and Mn metabolism [7].Concern about the potential for an additional manifestation of Mn neurotoxicity other than classical manganism was first raised by a study reporting that among 953 newly diagnosed cases of PD, the age at diagnoses was 17 years earlier in 15 career welders than non-welders. This “untypical” Mn-related neurotoxicity could be explained by findings that a career-mediated brain influx and a diffusion-mediated efflux caused Mn overloading the brain with prolonged excessive exposure and prolonged very low-level exposure. Based on these recent epidemiological studies, a concept of lifetime Mn exposure was developed with the hypothesis of an increased risk of Parkinsonian disturbances, where lifetime exposure to low Mn levels, starting from prenatal to older age, may be a risk factor for Parkinsonism. However, the mechanisms of Mn neurotoxicity at chronic low-level exposure are not sufficiently known yet. Consequently, these authors also drew attention to the need considering impaired liver function as being important for Mn related neurotoxicity as well as investigations whether GABAergic (gamma-aminobutyric acid) neurons of glutamate transport were affected by specific Mn species [7, 32].In another study, researchers compared Parkinsonian patients with non-Parkinsonian patients (controls) and concluded that exposure to Mn during life can increase the risk of neurodegenerative disorders via metal [Copper (Cu), Iron (Fe), Zinc (Zn)] concentration imbalance, especially when accompanied by a subclinical liver dysfunction. Investigations comprised of the associations between PD and exposure to industrial emissions of Mn as well as vehicle exhaust due to the use of MMT added to Canadian gasoline since 1976. According to the authors, the odds ratio for a physician-diagnosed PD was 1.034 per 10 ng/m3 increase in Mn in total suspended particles. Therefore, the researchers concluded that exposure to ambient Mn advances the age of PD diagnosis, thus strengthening the hypothesis that exposure to Mn adds to the natural loss of neurons attributable to the ageing process. These findings and conclusions from the researchers were in line with an earlier hypothesis of an increased risk of Parkinsonian disturbances after lifetime Mn exposure, expressed by the researchers [7].In the case of children, a 2009 study in Valcamonica, Italy showed that an impairment of olfactory function and motor coordination in different age groups like children and elderly might be caused by Mn due to transport of Mn through the olfactory tract leading to dopaminergic dysregulation. The effects of those environmental high concentrations of Mn in Valcamonica were also interesting regarding the younger population. Therefore, the researchers carried out neurobehavioral testing in adolescents (age 11-14), who had been living in Valcamonica. According to the authors, those pupils had a significant impairment of motor coordination, hand dexterity and odor identification, which were associated with soil Mn. Furthermore, tremor intensity was positively associated with blood and hair Mn. These data reinforce the fact that, also historical environmental exposure to Mn from ferroalloy emission could lead to olfactory and motor dysfunction in adolescence [7, 33].The effects of Mn in drinking water on children were further tested in a study in Quebec, Canada. The researchers found out that Mn intake by tap water was positively correlated with the impairment of school children at the age of 6 – 13 years. For example, a 10-fold increase of Mn in water was associated with a decrease of 2.4 IQ points (p<0.01), with a median Mn concentration in tap water of 34µg/L (range: 1-2700 µg/L) [7].For infant population, due to increased permeability of neuronal barriers and a decreased biliary excretion, younger individuals such as newborns are even at elevated risk and hence studies on Mn exposure on them is inevitable. A group conducted one of few infant studies on Mn exposure. Here the relationship between maternal and umbilical cord blood Mn levels and birth weight in a cohort of 470-mother-infant pairs born in Ottawa County, Oklahoma, was tested [7, 34]. In the study, maternal blood Mn levels during pregnancy were associated with birth weight in full-term infants in a non-linear pattern. Birth weight increased with Mn levels up to 3.1 µg/L, followed by a slight reduction in birth weight at higher levels. Thus implementation of such a study in higher exposed populations was recommended to find a clear correlation pattern. It is interesting in this context that the Mn blood status of pregnant women seems to be elevated due to physiological reasons [7, 35]. Concerning this topic, researchers tried to correlate maternal Mn levels with exposure levels of their breast-fed infants. The study was carried out in an area of Bangladesh, where water Mn levels exceeded the World Health Organization (WHO) guideline level by about 40%. Urine concentrations of the mothers correlated with water Mn concentration, but not blood or breast milk. Interestingly, elevated maternal Mn exposure did not necessarily lead to excessive exposure to breast-fed infants. This is why the authors stressed the importance of breast-feeding also in high Mn areas. Research shows that brain needs Mn during the early phases of development for important metalloenzymes, such as arginase, glutamine synthetase, pyruvate carboxylase, and superoxide dismutase [7, 8]. The influence of exposure to multiple chemicals already in early childhood was focus of a study by a group of researchers. In a longitudinal study in Mexico City, 455 children were enrolled at birth and followed until 36 months of age providing blood samples for measurement of Pb and Mn. Evidence of synergism between Pb and Mn was observed, whereby Pb toxicity was increased among children with high Mn co-exposure [7].The researchers suggested that joint exposure to both metals was associated with greater deficits, both in mental and psychomotor development, than effects of exposure to either mental alone. According to these authors, the age of 12 months is a sensitive developmental window specific to this Pb-Mn interaction, as it was only observed for this age but not for 24-month. The authors stressed the importance of including joint impact of chemicals in risk assessment and public health interventions, especially in areas where environmental Mn and Pb occur together [7].For post-occupational population, effects of occupational Mn exposure long after an ongoing employment with respiratory exposure to a certain amount of Mn are rarely documented. In view of this, a group of researchers carried out a followed-up study of the year 1990 in 2004 for workers who were exposed to Mn during their former working life in Quebec, Canada. The results suggested that past exposure to Mn may have lasting consequences on neuropsychiatric symptoms as those workers showed higher scales for anxiety, hostility and depression compared to a control group of workers not exposed to Mn. These findings locate the focus of Mn intoxication on other neurological effects than injury of neurons, but toward psychological effects and emphasized the danger of Mn still after a long time of acute exposure. Thus, it is known nowadays that neurotoxicity holds a time variable consistent of two parameters: the exposure duration as well as the period of life when it occurs [7].Additional work by other researchers measured inhalation exposure to Mn for a group of garage mechanics and a control group of nonautomotive workers. The airborne Mn exposure of 35 garage mechanics suspected of being relatively highly exposed to Mn from MMT was measured at the workplace over a one-week period. It was also measured for 30 nonautomotive workers at the University of Montreal. The environmental exposure was also measured for the two groups, as was the exposure to three other metals, Aluminum, Iron, and Zinc. At work, the mechanics were exposed to Mn concentrations varying from 0.010 to 6.673 µg/m3 with a mean of 0.45 µg/m3, while the control group was exposed to concentrations varying from 0.011 to 1.862 µg/m3 with a mean of 0.04 µg/m3. The mean environmental exposure for the two groups was similar to the Mn concentrations gathered in Montreal in 1992. Workplace concentrations of Al, Fe, and Zn were also higher for the garage mechanics. The result suggests that less than 10% of the Mn exposure of the garage mechanics was due to MMT. The levels of the metals measured were below the established limits for industrial and even environmental exposure [36].The majority of studies on the effects of exposure to high levels of Mn had been carried out at the workplace, in industries producing ferromanganese alloys and dry cells. These studies had shown notably that chronic exposure may affect the central nervous system and the respiratory system [31, 36].Other studies [37] conducted suggested that, iron compounds inhaled from the combustion of fuel containing ferrocene, act as local irritant to the lungs and gastrointestinal tract. Further, symptoms of overexposure to iron include irritation of eyes, mucous membranes, respiratory system, headache, dizziness, nausea, vomiting, fever, cyanosis, cough, dyspnea, liver, kidney, degenerative central nervous system. [37]
6. Octane Boosting Effectiveness
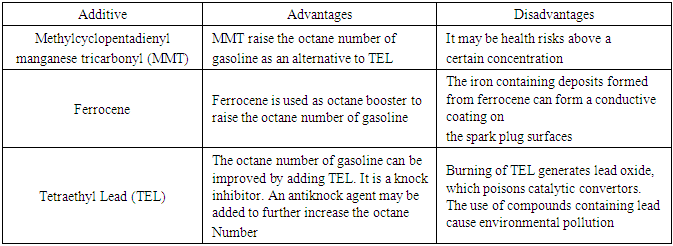
- Gasoline additives increase gasoline’s octane rating or act as corrosive inhibitors, thus allowing the use of higher compression ratios for greater efficiency and power. Octane boosters such as MMT, ferrocene and tetraethyl lead (TEL) are examples of gasoline additives. In particular, ferrocene used to raise the octane number of gasoline, is a cheaper alternative to MMT and is also used as an alternative to TEL by fuel refineries [38]. Table 2 shows the advantages and disadvantages of these gasoline octane boosting additives.
|
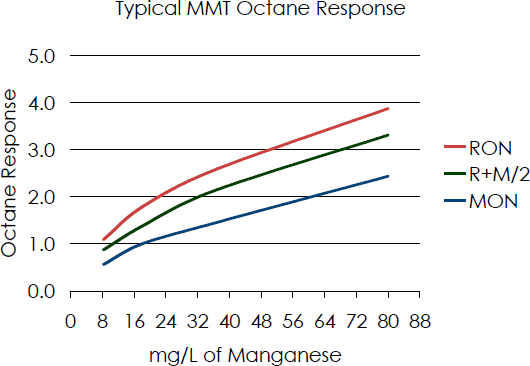 | Figure 5. Relationship between octane gain and manganese concentrations in fuel when using MMT [1] |
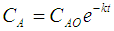 Where:CA= manganese concentration, mg/LCAO= initial manganese concentration prior to sunlight exposure, mg/Lk= reaction rate constantt= exposure period, min.• When exposed to sunlight, the decomposition of MMT resulted in the loss of octane number, increased existent gum and decreased induction period of the gasoline.• The MMT gasoline should be prevented from light exposure, especially direct sunlight. For MMT gasoline in China, the maximum exposure period should be less than 15 seconds to avoid the reduction of gasoline quality [39].Further, to confirm the effect of ferrocene on octane gain, the octane number of gasoline with ferrocene added was measured. To investigate the influence of ferrocene on engine, the deposit formation characteristics, the influence on the spark plug, and the influence on exhaust and fuel economy of the test vehicle were investigated by an engine bench test. The octane number (Research Octane Number and Motor Octane Number) of gasoline containing ferrocene was measured to confirm the octane number improvement. The octane number improvement was confirmed using the base gasoline with a different content of olefins. Low-octane gasoline was prepared by adding 12 vol. % hexane to regular gasoline. Low-octane-high-olefins gasoline was prepared by adding 12 vol. % hexane and 16 vol. % of 1-hexene to regular gasoline. The reagent ferrocene was used as an additive for evaluation. The ferrocene concentration was 20 mg/L and 40 mg/L in iron content. The iron content and octane number improvement of gasoline containing ferrocene are presented in figure 6. In the base gasoline, the effect of olefins on octane number improvement was not observed in this investigation. The octane number improvement decreased with increasing ferrocene concentration. Consideration of the reference data and trend of the low-octane-high-olefins data, a non-linear trend like dotted line in figure 6 is assumed [23].
Where:CA= manganese concentration, mg/LCAO= initial manganese concentration prior to sunlight exposure, mg/Lk= reaction rate constantt= exposure period, min.• When exposed to sunlight, the decomposition of MMT resulted in the loss of octane number, increased existent gum and decreased induction period of the gasoline.• The MMT gasoline should be prevented from light exposure, especially direct sunlight. For MMT gasoline in China, the maximum exposure period should be less than 15 seconds to avoid the reduction of gasoline quality [39].Further, to confirm the effect of ferrocene on octane gain, the octane number of gasoline with ferrocene added was measured. To investigate the influence of ferrocene on engine, the deposit formation characteristics, the influence on the spark plug, and the influence on exhaust and fuel economy of the test vehicle were investigated by an engine bench test. The octane number (Research Octane Number and Motor Octane Number) of gasoline containing ferrocene was measured to confirm the octane number improvement. The octane number improvement was confirmed using the base gasoline with a different content of olefins. Low-octane gasoline was prepared by adding 12 vol. % hexane to regular gasoline. Low-octane-high-olefins gasoline was prepared by adding 12 vol. % hexane and 16 vol. % of 1-hexene to regular gasoline. The reagent ferrocene was used as an additive for evaluation. The ferrocene concentration was 20 mg/L and 40 mg/L in iron content. The iron content and octane number improvement of gasoline containing ferrocene are presented in figure 6. In the base gasoline, the effect of olefins on octane number improvement was not observed in this investigation. The octane number improvement decreased with increasing ferrocene concentration. Consideration of the reference data and trend of the low-octane-high-olefins data, a non-linear trend like dotted line in figure 6 is assumed [23].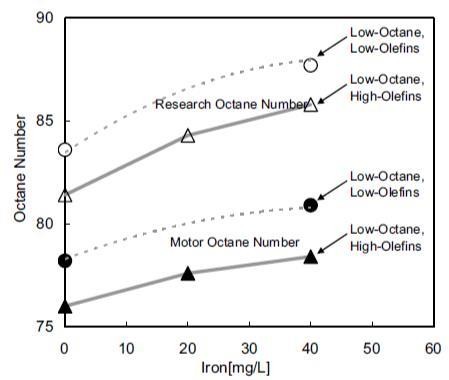 | Figure 6. Iron content and octane number improvement of gasoline containing ferrocene [23] |
7. Claimed Benefits: Air Quality and Fuel Economy
- Utilization of MMT allows refiners to use those refinery streams that possess clean-burning properties but have lower octane ratings. This can have beneficial effects of lowering fuel aromatic and olefin content as well as drivability index (DI). Reductions in these properties had generally been associated with the formulation of clean-burning fuel and reduced Mobile Source Air Toxics (MSAT) such as benzene. The use of the gasoline additive, MMT, permits refiners to beneficially alter fuel composition. In four significantly different fuel types, changes in fuel formulation resulted in a significant reduction in tailpipe emissions as compared to fuel not formulated with the additive. Emission changes observed with the use of fuels containing MMT were consistent with changes in fuel hydrocarbon composition [40].According to Ethyl studies [40], the use of MMT in gasoline also provides added protection to exhaust system components, particularly exhaust catalysts. Extensive testing demonstrated that vehicles operating on MMT containing fuel displayed greater overall emission system durability and higher exhaust catalyst efficiency than vehicles that operated on fuels without MMT. This testing and extensive use throughout the world demonstrated that the use of MMT additive is also compatible with all engine components and diagnostic systems. With the use of MMT formulated fuels, an overall effect of improved emission system durability and cleaner burning gasoline was achieved. The combination resulted in a 6% reduction in THC emissions, CO emissions reduction of 29%, 14% lower NOx emissions, 17% lower benzene emissions, and 21% and 30% lower emissions of N2O and NH3 respectively, compared to the use of a non-MMT fuel [40].In a study by Ethyl [40], vehicle emission system performance and durability of three fleets that accumulated 80,000 km per vehicle using either MMT formulated fuel or one of two non-MMT formulated fuels with similar properties was evaluated. Fleet emission system performance was assessed based on emission levels at the beginning and the end of the accumulation period. As a simple model to assess use of MMT versus non-MMT fuels in general, comparisons were drawn between vehicles using MMT formulated fuel and the combined fleet of vehicles using two non-MMT fuels.In an additional study by Ethyl [40], the effect of the two non-MMT fuels on emission system durability was also assessed. The conclusion drawn from this study was that, emission system performance was better with the MMT fuel compared to the non-MMT formulated fuels and that the two non-MMT fuels showed different emissions deterioration behavior. A surprising result was that emission control system performance degraded differently with the two different non-MMT fuels with essentially the same sulfur content. The fact that changes in typical fuel properties could impact emission system deterioration should be considered when looking at emission system performance with fuels formulated with MMT. Additionally, the instantaneous emission impact of fuels formulated with MMT was compared to similar fuels that did not contain the fuel additive. The conclusion arrived here was that gasoline could be formulated with MMT to provide lower automotive emissions than could be obtained from gasoline using other non-MMT blending components to enhance octane [40].Significant wear occurs in the exhaust valve seat with unleaded gasoline. The lead additive, in addition to its primary purpose of increasing octane quality, also provides a critical wear-reducing function by depositing a thin protective layer of lead salts on valve seat surfaces. Without this protection, poor valve seat sealing and loss of compression could occur and in turn result in loss of power, increased fuel consumption, rough engine operation, poor starting and increase in emissions, and ultimately severe engine damage. These problems can be overcome by the use of additive chemistries based on potassium, phosphorus or manganese that will prevent direct metal-to-metal contact that would otherwise cause high wear. The use of these additives at levels less than 50 mg/kg would especially keep older vehicles running. It was assumed that small amounts of Mn added to the environment by the combustion of MMT used as a fuel additive would be comparable to the normal background and should not create health problems. Because MMT has a low vapor pressure and a short half-life in sunlight, it is unlikely that significant concentrations of MMT could occur in the environment as a result of its use as a gasoline additive but manganese particles remain and do not disappear in combination with sunlight. Yet, it was reported that in context of a notable decrease in manganese emissions from industrial sources and the fact that atmospheric Mn pollution index seems to be stable over time, suggest that the combustion of MMT used in gasoline may be an important factor contributing to maintaining stable atmospheric Mn concentrations [41, 42].Since the MMT additive is used in parts per million (ppm) quantities versus the need to use other octane blending streams at levels of up to 10 to 15vol. %, refiners can increase gasoline octane quality without impacting bulk fuel properties. In the fuel formulation process, the use of MMT provides refiners with an energy efficient method to meet octane specifications and added flexibility to meet ever-tightening fuel specifications. To meet a target octane rating, MMT provides octane improvement in the full range of gasoline blend components. Use of the MMT fuel additive is compatible with octane blending agents such as oxygenates, MTBE and ethanol, and provides an octane boost in addition to that achieved with the oxygenates [42].Ethyl Corporation claimed that MMT maintained significantly higher catalyst conversion efficiency over the life of the catalyst converter. The mode of action seemed to be by protecting the catalyst against the slow degradation and poisoning by other components such as phosphorus and zinc. This was the complete contrast to lead, which irreversibly poisoned catalysts [16]. The CRC Data Analysis Panel hypothesized that the observed increase in catalytic converter efficiency with MMT fuels may be attributed to: • The change in feed gas composition with the MMT fuel or enhancement of the catalyst by manganese oxide deposition.• The increase in catalytic converter efficiency with MMT fuels in relation to clear fuel appeared to be linear with MMT concentration up to 15,000miles.• The increase in catalytic converter efficiencies with 31.25 and 62.50 mg Mn/gal MMT fuels in relation to clear fuel appeared to be about the same at 50,000 miles.• Conclusion bullets points 2 and 3 indicate that enhancement of catalytic converter efficiency occurred with MMT fuel. This is confirmed by other reported data [43].In Germany, commercial use of metal-containing gasoline additives was restricted by the Gasoline Lead Act, after the toxic effect of tetraethyl lead became evident. In a test program [44] with two pairs of technically identical vehicles, 15 ppm ferrocene as a gasoline additive proved to enhance octane, to reduce fuel consumption, and to result in lower pollutant emissions. Particulate emission levels were low and the iron content of the particles was only slightly changed [44, 45]. As the use of ferrocene as gasoline additive seemed to result in ecological and economic benefits, a testing program was set up by an expert panel convoked by the German Environment Agency (Unweltbundesamt-UBA) aimed at the investigation of differences in the toxicological properties of gasoline engine exhausts derived from fuel with and without ferrocene. This program included intensive chemical and physical investigations of the exhausts, a combined inhalation study on chronic toxic and carcinogenic effects of the exhausts, and in vitro studies on mutagenic, cytotoxic, and genotoxic effects of the condensates, particles, and the gaseous phase of the exhausts. In the case that no differences in the toxicity of the exhausts from fuel without and with ferrocene could be detected, a special license for ferrocene as a gasoline additive was expected. The procedure and results of the inhalation study were reported. With regards to the discussion of carcinogenic effects from solid particles, the formation of iron oxide (Fe2O3) particles was seen as a special problem. Because particle emissions of modern gasoline engines were very low compared to older engines which were fed with leaded fuel, and because the additional particle emissions from 15 ppm ferrocene were also very low (calculated: 450 mg/m3), it was decided to conduct all future investigations with twice the ferrocene concentration of the test program just mentioned (30 ppm), for better worked out possible effects from these particles [44].
8. Legislation: Europe, Continental, National
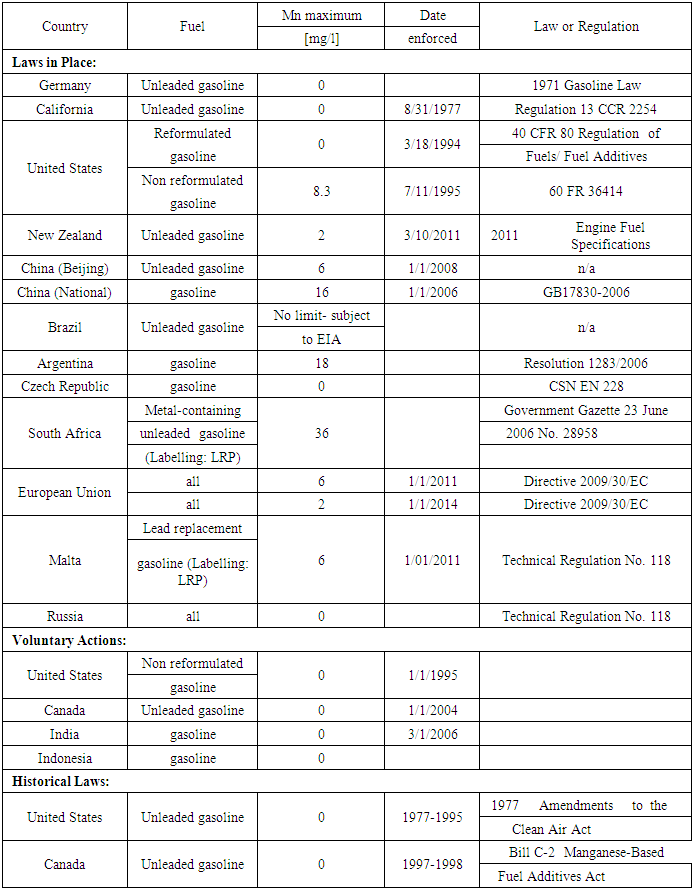
- The European Union implemented extensive regulatory programs intended to reduce emissions of pollutants from gasoline-powered vehicles. This is in response to concerns regarding the environmental impacts of these pollutants. The significantly stringent gasoline standard; EN 228 regulates fuel parameters but do not address certain fuel efficiency technologies that are considered “technical”, and Euro 3 & 4 and now Euro 5 & 6 require new vehicle technologies that must comply with these standards in customer service for periods of 160,000 kilometers or more and must be equipped with on-board diagnostic (OBD) systems that alert operators to the presence of defects or malfunctions that increase emissions beyond certain regulated threshold throughout the life of the vehicle. The technology advancements that allow for compliance with new emission legislations included the incorporation of high-density close-coupled (HDCC) catalysts, which differ from earlier catalysts in as they have more catalyst cells per unit area. This increase in cell density significantly increases the active surface area of the catalyst while reducing the mass of the catalyst and therefore the time required achieving the operating temperature of the catalyst [8].In addition, catalyst formulations were modified so that they can routinely withstand temperatures in excess of 800°C for extended periods of time. These advancements have provided vehicle manufacturers with catalysts that can be placed closer to the engine which allows the catalyst to reach optimum operating temperature quickly after the engine is cold-started in order to achieve very low pollutant emissions during all modes of operation [8].In order to achieve compliance with current emission standards, the properties and composition of the fuel upon which a vehicle operates should be treated as an integral component of the vehicle emission control system during the design, testing, and routine operation of that system. Vehicle manufacturers treat the engine, after treatment system and fuel as a complete and inter-related system. The worldwide automotive industry released a worldwide fuel charter (WWFC) that sets standards to harmonize global fuel quality [8]. This charter recognized that for “category one” fuels, metallic (i.e. potassium-based) additives are needed for valve-seat protection in vehicles that are not equipped with exhaust catalysts. However, the WWFC strongly recommended the removal of metallic fuel additives to no-detectible levels for fuel used in catalyst equipped vehicles. The WWFC particularly refers to Fe, Mn and Pb.In North America and Europe during the late 1990s, new vehicles with advanced emissions control systems began to be introduced. Studies [8] performed by the auto industry consistently found that the use of MMT in gasoline led to vehicle problems that included increases in engine-out hydrocarbon (HC) emissions, sparkplug misfire, exhaust valve leakage, varying degrees of catalyst plugging, increases in tailpipe emissions and/or exceedances of applicable emission standards. The auto industry studies also indicated that vehicles designed with the most sophisticated emission control systems were most susceptible to being adversely affected by the use of gasoline containing MMT. The studies conducted by Afton [8] supported to demonstrate either that the use of MMT in gasoline was benign, or that it improved catalyst performance to some degree and /or reduced certain emissions. However, recent studies by Afton as well as some Ethyl studies dating back to the 1970s demonstrated that MMT can lead to catalyst plugging [8]. Additionally, the application of metallic additives (e.g. Manganese) in European fuels meant that compliance with the emissions standards of today and of the future could no longer be guaranteed for advanced catalytic converters and the emission control system. Fundamentally, the stringent European emissions legislation demands the use of clean fuels. The presence of metallic additives in fuel contradicts this demand [8]. Table 3 shows the legal status of MMT use in selected countries around the world.
|
9. Current Gasoline Specification Limits
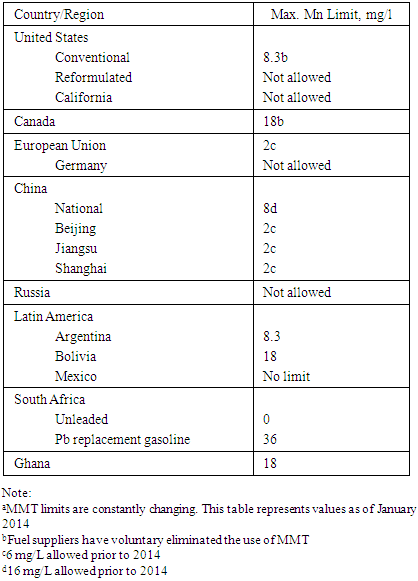
- The current gasoline specification limit for manganese in MMT is shown in Table 4. In Ghana, the manganese limit in MMT is 18 mg Mn/L [46]. In the case of ferrocene additive, a test program with two pairs of technically identical vehicles, in Germany, used a 15ppm ferrocene limit, though commercial use of metal-containing gasoline additives was restricted by the Gasoline Lead Act [44]. Table 4 shows reported 2014 MMT limits in gasoline from selected countries.
|
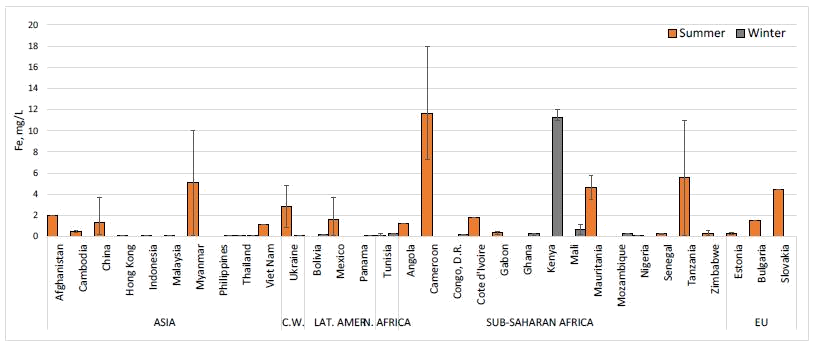
10. Current Market Penetrations
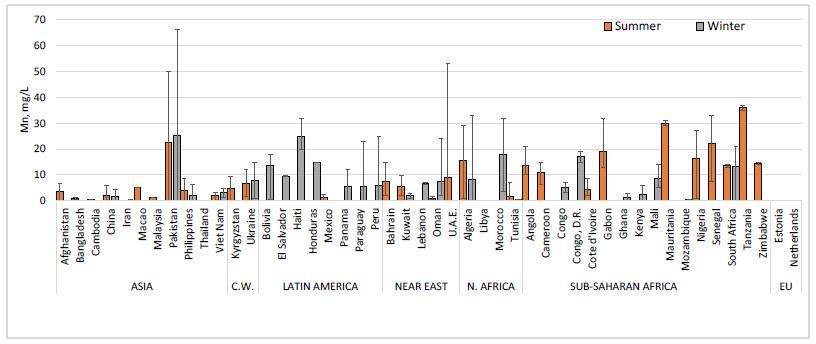
- Fuel surveys information, indicates that with the exception of a handful of countries, iron is rarely added to gasoline fuels. Iron as ferrocene is marketed as an antiknock by some additive producers in China and Canada. However, it does not appear that there is evidence of broad use of ferrocene in the gasoline pool [47]. The usage of metallic fuel additives in market place fuels is difficult to garner from literature sources, so fuel survey data from summer of 2015 and winter of 2015/2016 was acquired from SGC Fuel Survey [47]. Manganese was detected more frequently and in higher concentrations than any other element in both summer and winter, reaching as high as 91 mg/kg (66 mg/L) in the winter season, although the average dosing was much lower. In total, Mn was seen in 267 fuel samples collected from 48 different countries, with some seasonal variation for certain locations. Regional summary of manganese content, reported as mg/L, is shown in Figure 7, with the bars representing the average of all measurements above detection limits, and the error bars representing the minimum and maximum concentrations within a country [47].
11. Economic and Health Cost of MMT
- A new study [49] highlighted the potential human health and vehicle impacts associated with MMT use. With each of these impacts came an economic cost. For example, the health cost due to elevated blood lead levels that were a consequence of adding tetraethyl lead to gasoline amounted to approximately $172 billion annually [49]. This did not include the cost of health impacts associated with conventional pollutants, which were the initial target in the introduction of unleaded gasoline. The health impacts, and costs associated with use of MMT as a gasoline additive also included both direct manganese emissions and increased emissions of HC, CO, NOx, and particulate matter (PM). These conventional pollutants were associated with health impacts such as premature mortality, asthma, and cardiopulmonary diseases. Additional vehicle-related costs included reduced fuel economy and increased warranty and/or replacement costs of fouled spark plugs, plugged catalysts, poisoned sensors, and other vehicle components affected by MMT use. [49]. Furthermore, the actual costs (economic and health related) of not using MMT were quite low. The Environment Ministry of Canada determined that the additional cost to consumers of not using MMT was roughly 0.2 CAD cents/liter or 0.6 USD cents /gallon. If the fuel economy findings in the Alliance of Automobile Manufacturers (AAM) study proved to be robust, consumers would actually save at least twice that amount by not using MMT. Against a fuel economy penalty of 2%, the savings associated with not using MMT would be approximately 1 to 3 USD cents/gallon (0.3 – 0.8 USD cents/liter). The availability of reasonable, cost-effective alternatives suggests that nations should exercise caution regarding the public health and economic risks which may be posed by MMT use [49].While MMT is one of the lowest-cost octane enhancing additives after lead, costs for additional refining or replacement with more benign alternatives are not excessive. In Canada, where MMT use has been very widespread, a study commissioned by the Canadian environment ministers [49] determined that the cost to remove MMT from all gasoline in Canada would translate into an additional fuel cost of approximately 0.2 CA cents/liter or 0.6 US cents/gallon. This amounts to less than half of 1% of the current retail price in the US and is well within the typical market fluctuations for gasoline prices, which over the last two decades had fluctuated daily on the U.S. spot market by an average of 1 cent/gallon. In Canada, MMT was blamed for higher warranty costs and customers had complained of blocked and ineffective catalysts [49].
12. Stakeholder’s Position
- A consensus within the health community against the use of MMT started forming since the 1990s and recent developments renewed calls for action. The World Health Organization (WHO), the United States government, the Canadian government and others have adopted risk-based standards for airborne manganese compounds, recognizing that without regulations this pollutant poses a threat to health. In addition, the US EPA has listed manganese compounds as hazardous air pollutants. The American Journal of Industrial Medicine in 2007 published the Brescia Declaration that called for an immediate halt in all nations to the addition of organic manganese to gasoline. The American Academy of Pediatrics which advises pediatric physicians and which played an important role in the policy debate to phase out leaded gasoline stated in 2003, “to permit addition of MMT to the US gasoline supply would not be prudent”, and recommended that, “prevention of exposure to the most toxic additives to gasoline, such as tetraethyl lead, MMT, and others is best achieved by government regulation or phasing out of these compounds” [1]. The Clean Air Initiative for Asian Cities in 2008 released a roadmap for cleaner fuels and vehicles in Asia that recommended use of the precautionary principle: “prominent health experts raised serious concerns regarding the potential adverse health effects of metallic additives such as MMT and ferrocene, along with their potential adverse impacts on vehicle emissions and emissions control systems components. Therefore, the environmentally responsible approach for Asian countries was to apply the precautionary principle for these metallic additives and to not use them until and unless the scientific and health studies show that they are safe” [1].A 2004 Ford Motor user’s manual said: “Your engine was not designed to use fuel or fuel additives with metallic compounds, including manganese-based additives. Repairs to correct the effects of using a fuel for which your vehicle was not designed to use may not be covered by your warranty”. Honda on its website for owners contained this statement: “Do not use gasoline containing MMT, this additive contaminates your engine components and exhaust emission control system, and can lead to a significant increase in emissions and a loss in performance and fuel economy. Damage caused by the use of fuels containing MMT may not be covered under warranty” [1].Recognizing this controversy, the international review included a detailed analysis of the US EPA’s court cases involving the approval of MMT for unleaded gasoline in the US in 1995, as well as the government of Canada’s decision to continue to allow the use of MMT in Canada in 1998. Following the resolution in 1998 on the restrictions on the use of MMT in Canada, Sasol decided there was a sufficient case to proceed with using MMT. An important contributing factor for this decision was the findings of a personal exposure study undertaken in Toronto, Canada, where almost 100% of the unleaded gasoline contained MMT. The conclusion of Health Canada based on the study - namely that airborne manganese resulting from combustion of MMT in gasoline powered vehicles did constitute a health risk – coupled with the fact that Canada used MMT for 20 years, had an important hearing on Sasol’s decision [48].As part of their overall assessment of alternatives to leaded fuel, Sasol undertook an initial techno-economic study of MMT. Completed in 1996, this study which included various specifications and performance tests indicated that MMT was a desirable option. In addition to this techno-economic study, a review of the international experiences with MMT was also conducted. This study showed that\ while the use of MMT was technically acceptable, it also highlighted that it was a controversial option, with international motor manufacturers and various environmental health Non-Governmental Organizations (NGOs) campaigning against its use [48]. While this international experience may be seen by some as constituting a sufficient basis for a reasonable decision based on sound science, for others there was still sufficient uncertainty and cause for concern to invoke the precautionary principle and to avoid using MMT pending further clarity. A number of organizations and individuals maintained that further studies are required on the health and environmental impacts of MMT [8].Today, health scientists are strongly opposed to the use of MMT. Combustion of MMT releases manganese, a potent neurotoxin when inhaled. Therefore, health scientists urge policy makers at all levels of government to adopt a position that is in the interest of public health and welfare. Motor vehicle manufacturers are not against the use of all fuel additives and actively encourage the use of anti-corrosion and detergent additives for proper operation of vehicles in service. However, as well as MMT, motor vehicle manufacturers do not recommend, approve or permit the use of any metallic additives, including Fe or Pb [8]. The Motor Industry believes that substantial emission system exists related to the use of metallic additives. Now that the catalyst poisons, lead and sulfur are finally being eliminated, it is believed that the potential addition of other metallic compounds to fuels is a retrograde step, having great risk to current and future European air quality strategies and vehicle technologies [10].Given this overwhelming body of information, automobile manufacturers remain extremely concerned about MMT’s impact, especially on the highly sensitive technologies that are being or would be used in markets around the world. Most major auto manufacturers’ state in their Owners Guides that they recommend against the use of MMT, advising further that any damaged caused by MMT may not be covered by the warranty [50]. The Worldwide Fuel Charter recommends against the use of ferrocene and Mn (as MMT) in gasoline applications. Fuel survey information indicates that with the exception of a handful of countries, iron is rarely added to gasoline fuels.Sasol, however, undertook a precautionary approach. This process involved:• Assessing alternatives – since the early 1990s, the technical department of Sasol examined the possible alternatives for non-lead octane boosters, subjecting each of these to a technical and economic assessment, and seeking to find an appropriate balance between environmental, health, socio-economic and financial considerations.• Entering into dialogue – Sasol stated that, in the absence of any regulatory requirement for stakeholder participation, it was committed to follow an inclusive and transparent process regarding the introduction of MMT in South Africa; this process involved a lengthy process of interaction with representatives from government, other oil companies, motor vehicle manufacturers, fuel retailers, and civil society bodies, during which a number of commitments to ongoing monitoring and research were made. • Undertaking ongoing research and monitoring – Sasol undertook a number of activities aimed at identifying the relevant risks associated with the use of MMT; this included an independent environmental health risk assessment, an environmental impact assessment for the dosing installation, an independent manganese exposure assessment, and various exhaust gas emission tests. Committing to biannually review of the dosage level and to implement possible corrective action based on objective and meaningful criteria for South Africa, as well as undertaking to withdraw MMT if it is proven to be a cause for concern.Despite these various activities, some interested parties suggested that these are not sufficient to constitute meaningful implementation of the precautionary principle, arguing for example that there are still too many uncertainties and too much potential harm regarding MMT and that further research is required [48].
13. Conclusions
- Automobile manufacturers remain extremely concerned about organometallic additives’ impact, especially on the highly sensitive technologies that are being or would be used in markets around the world. The Worldwide Fuel Charter recommends against the use of ferrocene and Mn as MMT in gasoline applications.Regarding the information reviewed to date, the following points contributed to the final conclusion:• Canada stopped using MMT in 2004 based on the impact to health and vehicle engine components.• California and the other U.S states have voluntarily faced out MMT usage if not reduced it to a minimum of 8.3 mg Mn/L.• Auto manufacturers such as Ford and Honda, have taken a position where customer warranty claims could be refused if they use fuels containing MMT.• Health experts, including the World Health Organization (WHO), have also spoken up against the use of ferrocene and MMT due to their health challenges. For instance, health challenge such as manganism resulting when airborne manganese from combustion of gasoline congaing MMT is inhaled.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML