-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2017; 7(4): 114-119
doi:10.5923/j.ijee.20170704.02

Combined Production of Three Bioenergy Resources from Nannochloropsis sp. Microalgae
1Department of Civil and Environmental Engineering, University of Sharjah, Sharjah, United Arab Emirates
2Research Institute of Sciences and Engineering, University of Sharjah, Sharjah, United Arab Emirates
Correspondence to: N. Adam, Department of Civil and Environmental Engineering, University of Sharjah, Sharjah, United Arab Emirates.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, three bioenergy resources namely lipid, sugars and biogas were produced using two treatment sequences to combine production of three bioenergy resources from microalgae Nannochloropsis sp.,. Sequance-1 started with hydrolysis hydrocarbons using cellulase enzyme obtained from Trichoderma viride fungi. Following hydrolysis, lipids were extracted and the spent algae was mixed with wastewater sludge and anaerobically digested to produce biogas. Sequence-2 started with lipids extraction, followed by hydrolysis then biogas production. In sequence-1, hydrolysis improved with increased enzyme dose and produced a maximum of approximately 103 mg/g total sugars and 88 mg/g reducing sugars. Lipids were then extracted, which resulted in approximately 0.48 g lipids/g algae. Biogas production increased as the quantity of spent algae added to sludge increased but enzymatic hydrolysis reduced the substrate value of spent algae. The maximum specific biogas (263 mL/g) was produced from the water-hydrolyzed algae and sludge mixtures, then from the enzyme-hydrolyzed algae and sludge mixtures (213 mL/g), then from sludge alone (106 mL/g). In sequence-2, lipids (0.48 g/g) were fully extracted then enzymatic hydrolysis of the residues produced a maximum of approximately 83 mg /g total sugars and 79 mg/g reducing sugars. The ultimate biogas produced from the spent algae and sludge mixtures reached 238 mL/g. The results confirmed the potential of combining production of three bioenergy resources from Nannochloropsis sp. The sugars can be used for bioethanol production, the lipids for biodiesel production and the biogas can be processed to benefit from its methane content. Furthermore, the results suggested that the production sequence influences the relative production of the three resources, with sequence-1 being the better option.
Keywords: Microalgae, Nannochloropsis sp., Biofuel resources, Cellulase enzyme, Hydrolysis of hydrocarbons, Lipid extraction, Biogas production
Cite this paper: N. Adam, A. Shanableh, Combined Production of Three Bioenergy Resources from Nannochloropsis sp. Microalgae, International Journal of Energy Engineering, Vol. 7 No. 4, 2017, pp. 114-119. doi: 10.5923/j.ijee.20170704.02.
Article Outline
1. Introduction
- Cultivation of microalgae for production of bioenergy resources in sustainable manner has increased worldwide over the past decade [1]. Microalgae can serve as a non-edible biofuel feedstock alternative to fossil fuels [2, 3]. A key advantage for microalgae is the short harvesting cycle compared to conventional feedstocks [4, 5]. Furthermore, microalgae can be grown in many different environmental conditions unlike other biomass [6].Researchers [7-10] reported that several types of renewable biofuels can be generated from algae including: biodiesel, bioethanol, biogas, biohydrogen, syngas, and others. In recent years, Nannochloropsis sp. has been proposed as an excellent candidate for biofuels production due to its high lipid, which can reach up to 68% of dry weigh, as well as its moderate carbohydrates content [11-13].The major challenges of producing biofuels from algae relate to growing the desired algal species in abundant and feasible manner, as well as extracting the desired resources from algae efficiently. Researchers are continuously searching for alternative methods to enhance biofuels production from algae. A variety of pre-treatment methods have been developed to lyse algae cell walls in order to enhance extraction of algae resources, such as lipids and sugars, to produce biofuels. The pre-treatment methods include: thermal treatment, enzymatic treatment, acid lysis, microwave treatment, use of electrolyte solutions, and ultrasonic treatment. These methods proved to have various degrees of success [14-18]. Microalgae cell disruption is a critical step in the production of biofuels from algae, as in general algae cell walls are thick and contain multiple layers [19, 20]. Enzymatic pretreatment is a promising alternative [21] for disrupting algae cell walls and hydrolysis of algae hydrocarbons. A variety of microorganisms such as fungi and bacteria have the ability to lysis cellulosic material into glucose monomers [22].Researches [23-26] have confirmed that biofuels like biodiesel can be produced from algae, and in particular from microalgae that contain high lipids compared to macroalgae and other conventional crops. However, the possibility of simultaneously producing more than one biofuel from algae has not been adequately investigated. The focus in this study therefore to attempt to produce three biofuels resources from a selected type of microalgae.Recent studies [27-30] have focused on the production of one type of biofuel (i.e., biodiesel; bioethanol) from edible feedstocks and lignocellulosic biomass, but no available studies combined the production of different biofuels such as, biodiesel, bioethanol, and biogas from the same feedstock. Therefore, this study was intended to consider the potential of combining the production of biofuels resources such as lipid and sugars in order to produce biodiesel, bioethanol, and biogas from microalgae. Combing the production of biodiesel, bioethanol, and biogas from algae offers a variety of potential advantageous, including maximizing the feasibility of biofuel production, and providing potential alternative bioenergy sources that are more suitable environmentally.The purpose of this study was to assess the potential of combining the production of three types of biofuel resources (i.e., lipids for biodiesel, sugars for bioethanol, and algae residues for biogas production) from Nannochloropsis sp. microalgae. In particular, the study involved assessing two treatment sequences to assess their potential for producing the three desired biofuel resources.
2. Material and Methods
- The microalgae (Nannochloropsis Sp.) samples used in this study were purchased from Longevity Herbs and Superfoods, USA. The algae samples were washed, oven dried and grounded into a powder. The enzyme (referred to herein as CTV) used in this study was cellulase, which is obtained from Trichoderma viride fungi, was purchased from Sigma-Aldrich, USA with an activity of 3-10 units/mg. An enzyme solution was prepared by mixing 50 mg powder CTV enzyme in 50 mL sodium acetate buffer (100 mM, pH 5) in a glass bottle resulting in a 1 mg/mL solution. Two treatment sequences were used to produce the biofuel resources from the algae. Sequance-1 started with hydrolysis of algae hydrocarbons using the cellulase enzyme then lipids were extracted and the spent algae was mixed with wastewater sludge and anaerobically digested to produce biogas. Sequence-2 started with lipids extraction, followed by hydrolysis then biogas production. Various solutions were used as media for hydrolysis, including: sodium acetate buffer (100 mM, pH 5) with CTV enzyme; sodium acetate buffer (100 mM, pH 5) without CTV enzyme; distilled water at pH 7, and distilled water at pH 5. Enzymatic hydrolysis proceeded by adding 50 mL hydrolysis solution to 100 mL glass bottle with screw cap then placing the bottle in a water bath incubator shaker (Clifton NE5-28D, UK) to warm the solution to the desired hydrolysis temperature (37°C). After warming 5 g raw algae (sequence-1) or lipids-extracted algae (sequence-2) and the desired CTV enzyme quantity were added to the bottle and the contents shaken in the water bath at 300 rpm for 6 hours. Enzymatic treatment was terminated by boiling the glass bottles containing enzyme immediately for exactly 5 minutes in a water bath at kept at 95-100°C. The bottles were then cooled to room temperature, filtered using fine strainer, and centrifuged (Centurion 8000, UK) at 6,000 rpm to settle and recover the solids residues for either lipids extraction or biogas production. The supernatants were used for measuring total sugars using the phenol-sulfuric acid method [31] and reducing sugar according to the DNSA method [32]. Duplicates and triplicates were used to ensure accuracy and to obtain enough residues for lipids extraction or biogas production. The experiment was repeated three times with nearly identical results and the average value was reported.The algae recovered following enzymatic hydrolysis in Sequence-1 were mixed together, soaked in distilled water for 12 hours, oven dried then milled into powder. On the other hand, the algae samples hydrolyzed without enzymes were not mixed and were kept separately. The lipids in the recovered algae were extracted first using the modified Bligh and Dyer method [33] then extraction was completed using the Soxhlet method with a mixture of chloroform, methanol, and deionized water (volume ratio of 1: 2: 0.8). In sequence-2, lipids were extracted from the raw algae using Soxhlet extraction only.Biogas production was carried out in 50 mL capped serum bottles and the produced biogas was measured weekly using a manometer for 16 weeks. Control samples containing raw algae 2% TS) were also digested. The serum bottles were filled with 30 mL sludge, and the pH was adjusted to approximately 7.3. Increasing quantities of dry algae samples were added to the 30 mL 2% sludge samples in the bottle to produce algae/sludge TS ratios of 0%, 10%, 20%, 30%, 40% and 50%. The serum bottles were covered with rubber, sealed with aluminum and incubated at 37°C in an incubator (GENLAB, UK). The biogas production experiments were conducted in triplicates with nearly identical results and the average value was reported.
3. Results and Discussion
- Microalgae Nannochloropsis sp. is known to be rich in lipids and the microalgae used in this study yielded a maximum of approximately 0.49 g lipids per g algae (i.e., 49% by weight). Furthermore, the maximum quantity of total sugars that was hydrolyzed from the algae using cellulose enzyme reached approximately 0.19 g/g, or 19%. The results of the two treatment sequences used in this study are discussed in the following subsections.
3.1. Enzymatic Hydrolysis
- Sequance-1 involved first enzymatic hydrolysis to produce sugars then lipids extraction then biogas production from mixtures of spent algae residues and wastewater sludge. Hydrolysis of algae carbohydrates (without cellulase) using distilled water at pH 5 or pH 7 or using the acetate buffer at pH 5, resulted in total sugars production of 39 mg/g algae to 42 mg/g algae. Likewise, hydrolysis without enzyme resulted in reducing sugars of approximately 34 mg/g algae to 35 mg/g algae, as shown in Figure 1(a). The results suggest the presence of significant quantities of readily hydrolysable carbohydrates. Using the CTV cellulase enzyme, the data in Figure 1(a) show that total sugar yield increased in the range of 77 mg/g to 103 mg/g. Similarly, the reducing sugars increased in the range of 72 mg/g algae to 88 mg/g with increasing enzyme use. Clearly, the CTV cellulase enzyme significantly increase sugars yield from the algae. Sequence-2 involved extracting lipids from raw algae then hydrolyzing the carbohydrate to produce sugars, then co- digesting the spent algae residues with sludge to produce biogas. Hydrolysis of the lipid-extracted algae without enzyme resulted in total sugars production of approximately 31 mg/g algae and reducing sugars of approximately 27 mg/g algae (Figure 1,b). Using three dosages of the CTV enzyme, the total sugar yield increased in the range of 47 mg/g algae to 83 mg/g algae and the reducing sugars yield increased in the range 44 mg/g algae to 79 mg/g algae (Figure 2). Compared to sequence 1, hydrolysis of the lipid-extracted algae in sequence-2 resulted in significant reduction in total sugars production (19%-39% reduction) and reducing sugars production (10%-39% reduction). Lipids extraction resulted in significant loss in the mass of raw algae (approximately 50%) and the sugars production results reported in Figure 1(b) are relative to the residual mass of algae after lipids extraction rather than relative to the original raw algae mass. If corrected for algae mass loss due to lipids extraction, the sugars production results would amount to about half the values reported in Figure 1(b). Therefore, lipids extraction apparently reduced the sugars extraction potential of algae, which may be attributed to loss of hydrolysable carbohydrates. Therefore, hydrolysis prior to lipids extraction proved to be more beneficial in terms of sugars production.
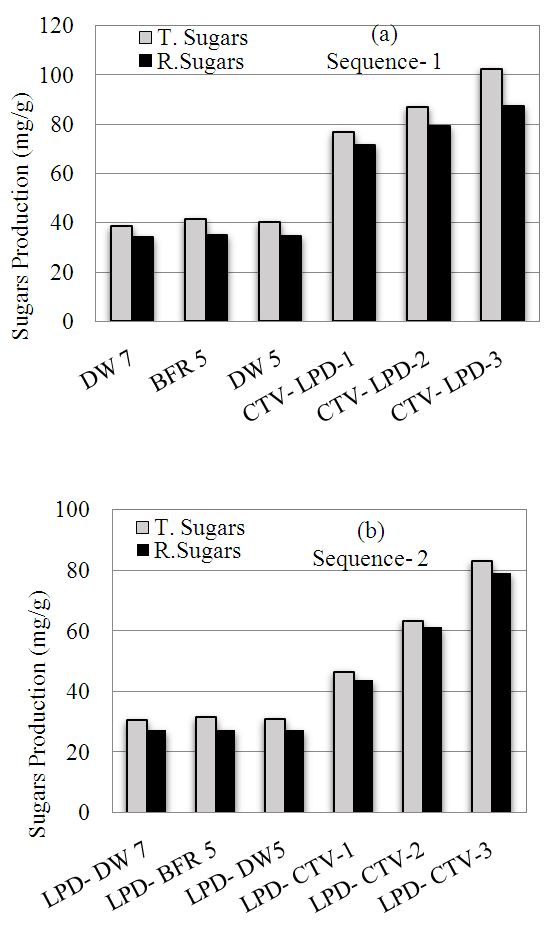 | Figure 1. Results of hydrolysis of Nannochloropsis sp. with and without cellulose enzyme to produce total and reducing sugars |
3.2. Lipids Extraction
- In sequence-1, lipids extraction was carried out in two steps. Step-1 involved extraction using the modified Bligh and Dyer method [34]; and step (2) involved Soxhlet extraction. The reason for using two extraction steps was to assess the impact of enzymatic hydrolysis on lipids extraction using the modified Bligh and Dyer method, which proved to be a weak extraction method when use to recover lipids from the raw algae. The lipids extraction results using the modified Bligh and Dyer method are shown in Figure 2. The results in Figure 2(a) show that without enzyme, the amount of lipids extracted amounted to about 10% of algae weight in the three control samples hydrolyzed with distilled water or the acetate buffer. The use of three enzyme dosages increased lipids yield to 14%-20% of algae mass. The increase in lipids extraction due to enzyme use can thus be attributed to the action of the enzyme, which typically involves degradation the walls of algae cells. It should be noted that in sequence-1, 24-35% of the algae mass was lost during enzymatic hydrolysis. Therefore, the lipid yield results reported in Figure 2(a) are relative to the residual algae following hydrolysis. The lipids yields corrected for mass loss due to enzymatic hydrolysis are shown in Figure 2(b). The corrected lipids yield amounted to 8% without enzyme use and 10%-13% with enzyme use. Clearly, the results suggest a small improvement in lipids extraction following enzymatic treatment.
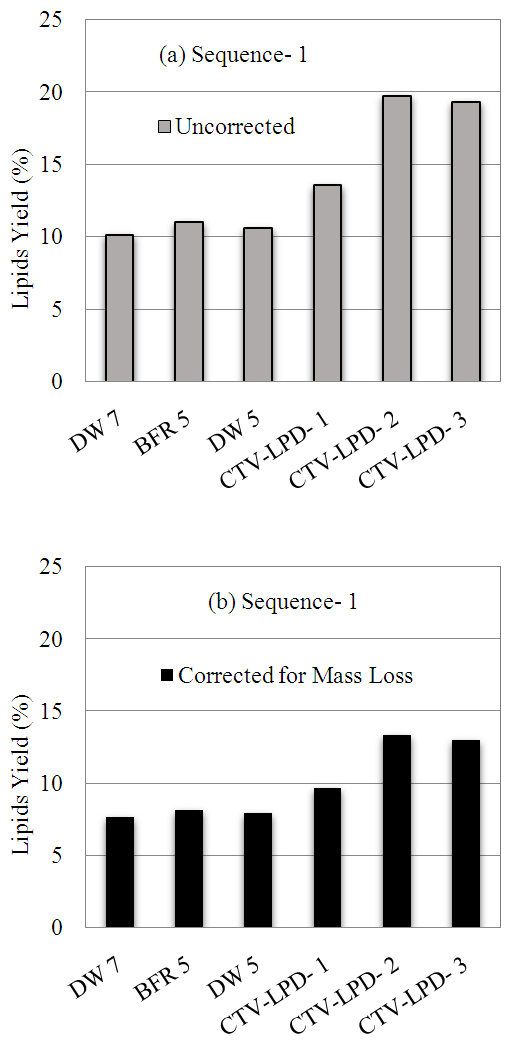 | Figure 2. Lipids yields from algae residual following enzymatic hydrolysis |
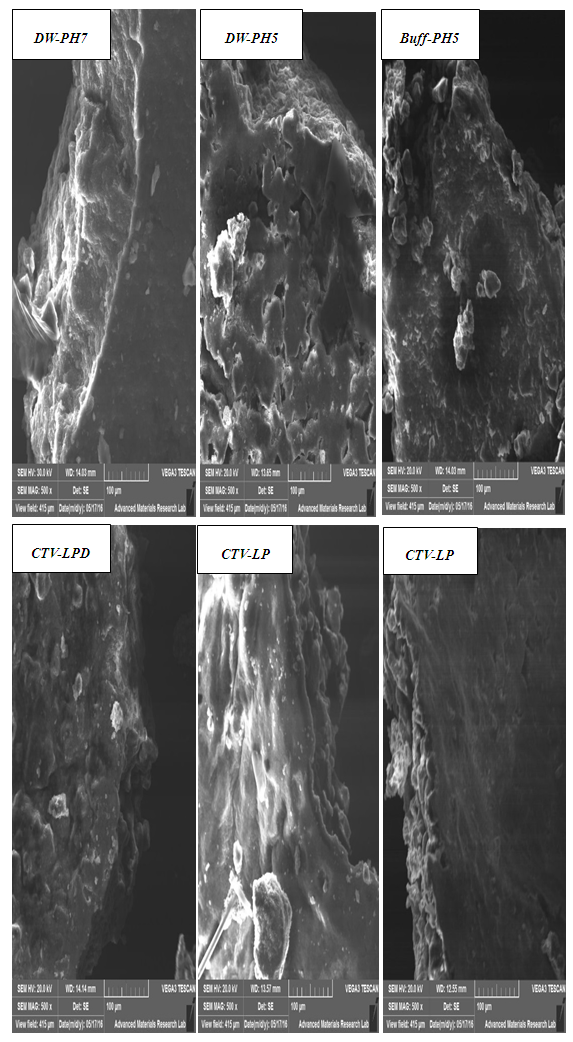 | Figure 3. SEM images of morphological analysis following algae hydrolysis |
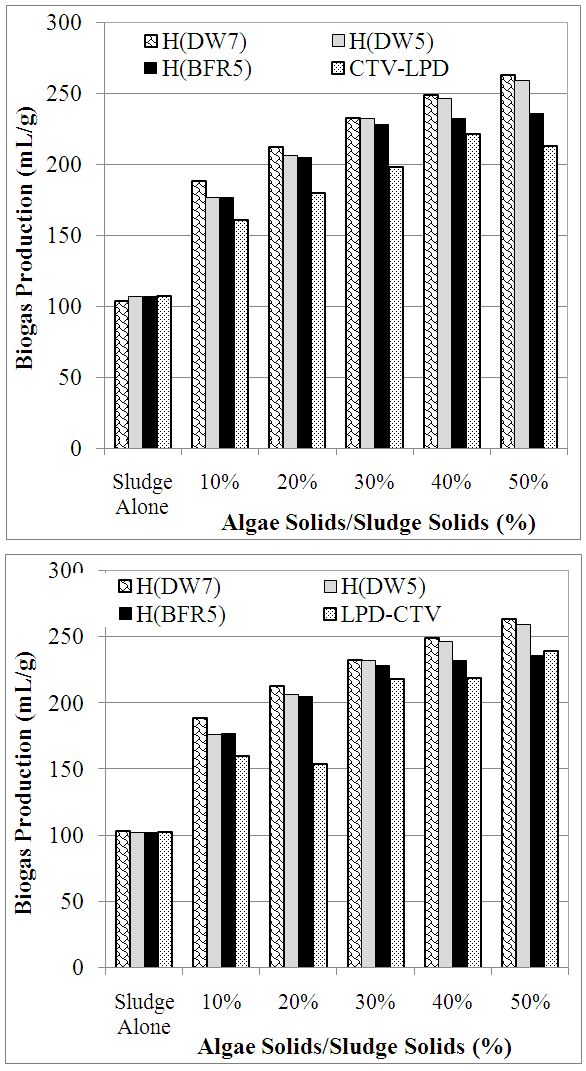
3.3. Biogas Production
- Figure 4(a) provide a comparison of specific biogas production (i.e., mL biogas/g solids) after 16 weeks of incubation from mixtures of sludge and algae subjected to sequence-1 treatment. The results show that the addition of up to 50% algae to sludge significantly enhanced specific biogas production by up to 2.5 times compared to sludge alone. Furthermore, the contribution of spent algae to biogas production increased as the quantity of spent algae mixed with sludge increased. Biogas production was highest from the mixtures that contained sludge and spent algae that was not subjected to enzymatic treatment. The highest biogas production was from the mixtures containing the algae hydrolyzed using distilled water at pH 7, then from the mixtures containing the algae hydrolyzed using either distilled water at pH 5 or buffer at pH5, and the least biogas production was from the mixtures containing enzymatically treated algae. The results clearly suggest that enzymatic hydrolysis resulted in loss of biogas-generating substrate from treated algae.The cumulative biogas production results for sequence-2 treatment are shown in Figure 4(b). The data show biogas production trends similar to those obtained for sequence-1 samples. Thee specific biogas production was in the range of 100 mL/g to 263 mL/g in the following order: distilled water (pH7)-hydrolyzed algae > distilled water (pH5)-hydrolyzed algae > buffer (pH5)-hydrolyzed algae > lipids extracted then enzyme-hydrolyzed algae > enzyme-hydrolyzed then lipids extracted algae > sludge alone. Biogas production thus reflected the substrate value of residual algae following hydrolysis and lipids extraction.
4. Summary and Conclusions
- In this study, three biofuel resources (i.e., lipids, sugars, and biogas) were produced from Nannochloropsis sp. microalgae. Enzymatic hydrolysis, lipid extraction and anaerobic digestion were used to produce the three resources using two treatment sequences: (a) hydrolysis, lipid extraction then biogas production, and (2) lipid extraction, hydrolysis then biogas production. In sequence-1, enzymatic hydrolysis produced a maximum of 103 mg/g total sugars and 88 mg/g reducing sugar compared to 42 mg/g total sugars and 35 mg/g algae produced without using enzymes. In sequence-2, a maximum of 83 mg/g total sugars and 79 mg/g reducing sugar compared to 32 mg/g total sugars and 27 mg/g reducing sugar algae produced without using enzymes. The hydrolysis results suggest the presence of significant quantities of readily hydrolysable carbohydrates. Furthermore, the results indicated that lipid extraction prior to enzymatic hydrolysis reduced sugars production. The SEM images demonstrated ultrastructural changes in algae morphology following enzymatic hydrolysis enzyme compared to the intact structure of raw algae. Following hydrolysis and lipid extraction, the spent algae provided a useful source of substrate for biogas production. Overall, the specific biogas production was in the range of 100 mL/g to 263 mL/g and was obtained in the following order from mixtures of sludge distilled water (pH7)-hydrolyzed algae > distilled water (pH5)-hydrolyzed algae > buffer (pH5)-hydrolyzed algae > lipids extracted then enzyme-hydrolyzed algae > enzyme-hydrolyzed then lipids extracted algae > sludge alone. The obtained results demonstrated the potential of producing three biofuel resources from Nannochloropsis sp.Production of biofuels from microalgae is challenging topic, but what makes it even more interesting is the possibility of combining the production of different biofuels from algae such as, biodiesel, bioethanol and biogas. The possibility of simultaneously producing more than one biofuel from algae has not been adequately investigated. That’s why focus in this study therefore was to attempt to produce three biofuels resources from a selected type of microalgae using enzymatic hydrolysis of cellulose as a Pretreatment alternative.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML