-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2014; 4(4): 75-80
doi:10.5923/j.ijee.20140404.02
A Review on Hydrogen as a Fuel for Automotive Application
Moses Omolayo Petinrin1, Moshood Bolaji Adebayo2, Adeniji Toyosi Adelowokan2
1Department of Mechanical Engineering, University of Ibadan, Ibadan, Nigeria
2Prototype Engineering Development Institute, National Agency for Science and Engineering Infrastructure, Ilesa, Osun State, Nigeria
Correspondence to: Moses Omolayo Petinrin, Department of Mechanical Engineering, University of Ibadan, Ibadan, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Hydrogen can be described as the best alternative to the conventional fuels used to power automobiles for several reasons, and these include its higher heating values and emission free combustion. It has been demonstrated as a viable automotive fuel in three technological modes: hydrogen internal combustion engines (HICEs) connected mechanically to conventional vehicles; fuel cells that produce electricity to power electric vehicles; and hybrids that involve combinations of engines or fuel cells with electrical storage systems, such as batteries. The main drawbacks of using hydrogen as a transportation fuel are huge on-board storage tanks. Hydrogen stores approximately 2.6 times more energy per unit mass than petrol but it needs an estimated 4 times more volume than petrol to store that energy. In this review, the properties and the pathways for hydrogen use in vehicles, factors affecting the commercialization of hydrogen vehicles, which are not limited to the production; storage; transportation and distribution of hydrogen; costs of production, storage and distribution; and its safety, were discussed.
Keywords: Emission, Fuel cell, Hybrid power, Automotive, Hydrogen commercialization
Cite this paper: Moses Omolayo Petinrin, Moshood Bolaji Adebayo, Adeniji Toyosi Adelowokan, A Review on Hydrogen as a Fuel for Automotive Application, International Journal of Energy Engineering, Vol. 4 No. 4, 2014, pp. 75-80. doi: 10.5923/j.ijee.20140404.02.
Article Outline
1. Introduction
- Over the years, majority of road vehicles have been powered by either petrol or diesel in internal combustion engines. The fuels have also undergone many changes to provide more power and less harmful emissions [1].One of the problems related with the combustion of petrol and diesel are the gases caused by incomplete combustion, carbon monoxide is a product of this incomplete combustion, which is highly poisonous [2-5]. Nitrogen oxides (NOx) are generated when nitrogen in the air reacts with oxygen under the high temperature and pressure conditions inside the engine. NOx emissions contribute to both smog and acid rain.These harmful emissions associated with fuels had been a great concern by the automotive engineers and this has led to some modifications and changes in the fuels used to power the automobile engines, and also the modifications and changes in the design of the gas-exchange system. Tremendous reduction in exhaust emission has been achieved by the addition of some components in the exhaust system like the catalytic converter, and the presence of the Exhaust Gas Recirculation, (EGR) Systems [6, 7].Emissions controls have been highly successful in reducing the emissions produced by motor vehicles in terms of quantity per distance travelled. However, substantial increase in the distance travel by vehicles, and equally substantial increase in the number of vehicles in use imply that the overall reduction in pollution is low.Carbondioxide (CO2) is a product of the complete combustion of hydrocarbons. The problem associated with CO2 is a global issue, being one of the greenhouse gases and a major contributor to global warming; throughout time CO2 produced by respiration in animals and plants has balanced with CO2 usage for photosynthesis in plants. In recent decades CO2 production has increased greatly due to burning fossil fuels, and the usage of CO2 has also decreased due to the felling of much rainforest trees. This has caused a greater concentration of CO2 in the atmosphere. The increasing concentration of CO2 is increasing the greenhouse effect and this is causing a global rise in average temperatures, which some say is to blame for the apparent increase in natural disasters.In recent years concerns have been raised about the suitability of petrol and diesel for predominant use in future road vehicles [8]. There are many reasons why we should be looking at alternative fuels for the future apart from the fuel emissions. The source of both petrol and diesel is crude oil, so when there is such demand for these fuels this places crude oil in high demand, with roughly half of the worlds processed crude oil fuelling our road vehicles. Crude oil is a non-renewable resource and some estimates state that at our current usage we have as little as 50 years supply left, these estimates are open to much discussion, but they still point to a diminishing supply, this possible fuel crisis provides an incentive to consider alternative ways to fuel the road vehicles [9].In this article, clear elucidation will be made on hydrogen as an alternative fuel for automotive application because of the intrinsic no air-polluting emission properties and its environmental performance.
2. Development of Hydrogen Vehicles
- According to [10, 11], hydrogen was the first fuel to be used in an internal combustion engine back in 1807; the engine was designed and built by Francois Isaac de Rivaz. The design was very unsuccessful because there were many safety issues; however, it never took off as a viable source of propulsion. Nearly 200 years on and things are looking very different, hydrogen is now offering emission-free motoring using very familiar technology. Hydrogen can be obtained from both renewable and non-renewable energy sources.When combustion of hydrogen occurs the only product is water, which is obviously completely harmless [12]. But the source of the hydrogen needs to be considered, and the most emission free way of obtaining hydrogen is by electrolysis of water (separating water into hydrogen and oxygen by passing a current through it). The most common power source for this is fossil fuels, which is the source of more than 99% of the hydrogen produced worldwide [13], so there are emissions, but far lower than the equivalent petrol or diesel powered internal combustion engine, due to the advanced emission control systems at power stations. However in the future we may obtain completely emission free motoring by powering the electrolysis with renewable sources [14, 15].Figure 1 shows the heating values of some selected fuels, these values are also referred to as calorific value of the fuel. The high heating values of hydrogen help in giving out required heat energy that can be converted to mechanical energy in an engine.
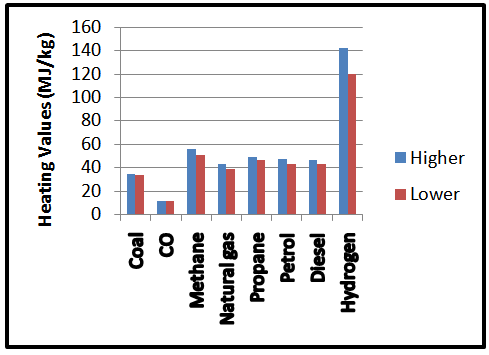 | Figure 1. The Higher and Lower Heating Values for Some Selected Fuels |
3. Pathways for Hydrogen Use in Vehicles
- Hydrogen has been demonstrated as a viable automotive fuel in three technological modes, namely: hydrogen internal combustion engines (HICEs) connected mechanically to conventional vehicles; fuel cells that produce electricity to power electric vehicles; and hybrids that involve combinations of engines or fuel cells with electrical storage systems, such as batteries [16].
3.1. Hydrogen in Internal Combustion Engines
- The hydrogen internal combustion engine car [17, 18] is a slightly modified version of the conventional petrol internal combustion engine car. The conventional engines require modification, not major redesigning, to burn hydrogen. The proven, commercially available technology to use natural gas in internal combustion engines is similar to the technology needed to use hydrogen. Hydrogen internal combustion cars burn hydrogen directly, with no other fuels and produce pure water vapour exhaust.A small proportion of hydrogen in an internal combustion engine can both increase overall efficiency and reduce pollution. Such a conventional car can employ an electrolyser to decompose water [19, 20], or a mixture of hydrogen and other gasses as produced in a reforming process. Since hydrogen can burn in a very wide range of air/fuel mixtures, a small amount of hydrogen can also be used to ignite various liquid fuels in existing internal combustion engines under extremely lean burning conditions. This process requires a number of modifications to existing engine air/fuel and timing controls.The primary shortcomings of these cars is the hydrogen fuel that can be stored in a normal size tank is used up rapidly. A full tank of hydrogen, in the gaseous state, would last only a few miles before the tank is empty. However, methods are being developed to reduce tank space, such as storing liquid hydrogen or using metal hydrides in the tank [21].
3.2. Hydrogen in Hybrid Power Source
- Demonstrations of hybrid technology involving hydrogen indicate that these vehicles may be lighter, smaller, more versatile, and offer better performance than vehicles running solely on hydrogen engines, fuel cells, or batteries. By combining on-board engines or fuel cells that generate power with electrical systems that store power, electric hybrids may offer greater market potential than vehicles powered solely by single systems.It is possible to use part of liquid fuel (petrol, diesel, or methanol) stored on-board a vehicle and then use an on-board reformer to separate the hydrogen just before it is used in the fuel cell. While this requires additional equipment on the vehicle, it removes the need for high-pressure gas storage. These different storage technologies can introduce significantly different potential hazards [22].
3.3. Hydrogen in Fuel Cells
- Fuel cells provide power in a similar way to batteries, a chemical reaction occurs within the cell which provides electricity to drive electric motors. The difference between a fuel cell and a battery is that the fuel is being continuously replaced from a tank this tank can then be refilled far faster than a battery will charge [12]. Although the majority of fuel cells use hydrogen as the fuel, while some uses methane, and a few use liquid fuels such as methanol.Fuel cells require no lubricating oil and there is no combustion to generate the high temperatures that lead to the formation of nitrogen oxides, fuel cell-powered electric vehicles offer the cleanest way of using hydrogen: they are emission-free vehicles [16, 23]. Fuel cells are two to three times as energy efficient as combustion engines [14]. An internal combustion engine loses more than 80% of the energy it generates, either as waste heat or friction. When a hydrogen fuel cell is used, the energy loss is 40% to 60%, so the percentage of energy that is delivered as movement is much greater.Fuel cells that use hydrogen can be thought of as devices that do the reverse of the well-known experiment where passing an electric current through water splits it up into hydrogen and oxygen. In the fuel cell hydrogen and oxygen are joined together to produce water and electricity. Hydrogen fuel is fed into the anode of the fuel cell, while oxygen (or air) enters the fuel cell through the cathode. With the help of a catalyst, the hydrogen atom splits or ionizes into a proton (H+) and an electron (e-), which takes different paths to the cathode. The proton passes through the electrolyte.Anode reaction:
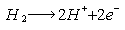 Cathode reaction:
Cathode reaction: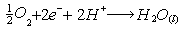 Overall reaction:
Overall reaction: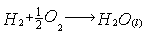 The Figure 2 shows the flow of energy through the vehicle.
The Figure 2 shows the flow of energy through the vehicle.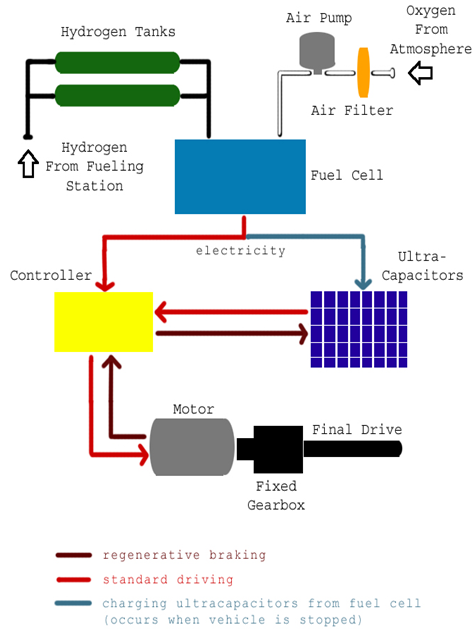 | Figure 2. The Schematic diagram of flow of energy through the vehicle [1] |
4. Factors Affecting Commercialization of Hydrogen Vehicles
4.1. Production
- Hydrogen produced today is made mainly from fossil fuels in a process known as steam reforming or partial oxidation of natural gas [13]. Because this is the cheapest and most firmly established method of producing hydrogen, it is likely to predominate until production technologies based on renewable energy resources become commercially competitive. All of the methods of producing hydrogen from renewable resources face technical and economic hurdles that must be overcome if hydrogen is to fuel a sustainable transportation economy [14].There is concern about the energy-consuming process of manufacturing the hydrogen. Manufacturing hydrogen requires a hydrogen carrier such as a fossil fuel or water. The former consumes the fossil resource and produces carbon dioxide, while electrolyzing water requires electricity, which is mostly generated at present using conventional fuels (fossil fuel or nuclear power). While alternative energy sources like wind and solar power could also be used, they are still more expensive given current prices of fossil fuels and nuclear energy. In this regard, hydrogen fuel itself cannot be called truly independent of fossil fuels (or completely non-polluting), unless a totally nuclear or renewable energy option were considered [24].
4.2. Storage
- Storing hydrogen on-board a vehicle raises three critical issues: the weight of the fuel storage system, the system's volume, and the speed or ease of refuelling the vehicle. Although molecular hydrogen has excellent energy density on a mass basis, as a gas at ambient conditions it has poor energy density per volume. As a result, if it is to be stored and used as fuel on-board the vehicle, molecular hydrogen must be pressurized or liquefied to provide sufficient driving range [21]. Increasing gas pressure improves the energy density per volume, making for smaller, but not lighter container tanks. Alternatively, higher volumetric energy density liquid hydrogen may be used.However, liquid hydrogen is cryogenic and boils at –252.88°C. Cryogenic storage cuts weight but requires large liquefaction energies. The liquefied hydrogen has lower energy density per volume than petrol by approximately a factor of four. Storage tanks must also be well insulated to minimize boil off.Hydrogen, as a small, energetic molecule, tends to diffuse through any liner material intended to contain it, leading to the embrittlement, or weakening, of its container. Insulation for liquid hydrogen tanks is usually expensive and delicate. The limitations of the tank weight and volume of hydrogen can also be minimized if it is used in fuel cells.The most common method of on-board hydrogen storage in today's demonstration vehicles is as a compressed gas at pressures of about 700bar (70MPa). Many people believe that the energy needed to compress hydrogen to these pressures presents a major barrier to a hydrogen economy [14, 21]. For example, if one considers the entire world using hydrogen just in their cars, then a large amount of energy would be needed simply to compress the hydrogen for storage, of the order of 30% of the total energy used for transport. If this energy was not recovered in any way, the net energy used to compress it would be wasted. A seemly ordinary hydrogen tank that can actually store up to 400 litres of Hydrogen by compressing it is as shown in Figure 3 [1].
 | Figure 3. An ordinary hydrogen tank [1] |
4.3. Transportation and Distribution of Hydrogen
- Hydrogen must be transported to markets once it is produced. Today's hydrogen distribution system is extremely limited: there are only 450 miles of hydrogen pipeline in the USA, compared with 200,000 miles of oil pipeline and 1.3 million miles of natural gas pipeline [14, 24, 27].Nonetheless, hydrogen pipeline distribution is a firmly established technology. The key obstacle to making the fuel widely available is the scale of expansion needed to serve transportation markets. Liquefy hydrogen is distributed via barges, tankers, and rail cars.
4.4. Costs of Production, Storage and Distribution
- Hydrogen requires huge investments in the infrastructure to produce, store and distribute it to vehicles, in addition to the cost of the new vehicles themselves.Hydrogen pipelines are more expensive than even long-distance electric lines. Hydrogen is about three times bulkier in volume than natural gas for the same energy delivered, and hydrogen accelerates the cracking of steel (hydrogen embrittlement), which increases maintenance costs, leakage rates, and material costs [14, 27].Hydrogen fuel cells were initially characterized by the high production costs associated with converting the gas to electricity ultimately required to power a hydrogen car. Scientists are currently studying how to produce inexpensive fuel cells that can be used on automobiles. Recently, alternative methods of creating hydrogen directly from sunlight and water through a metallic catalyst have been announced. This may provide a cheap, direct conversion of solar energy into hydrogen, a very clean solution for hydrogen production [28, 29].Although the cost of fuel cells have decreased significantly, the cost for a fuel cell system is almost double that of an internal combustion engine [30].
4.5. Environmental Concerns
- Hydrogen gas produced from fossil fuels, especially through the natural gas steam reforming method, creates carbon dioxide (CO2), a greenhouse gas, as a by-product. This is usually released into the atmosphere, although there has also been some research into production of less CO2 as a by-product [31].A very interesting study published by the Pembina Institute, based in Calgary, Alberta, compared total carbon dioxide emissions of fuel cell vehicles using hydrogen produced from a variety of methods (Figure 4). The results clearly show that the choice as to which method will be used to produce the hydrogen will be a critical environmental decision.
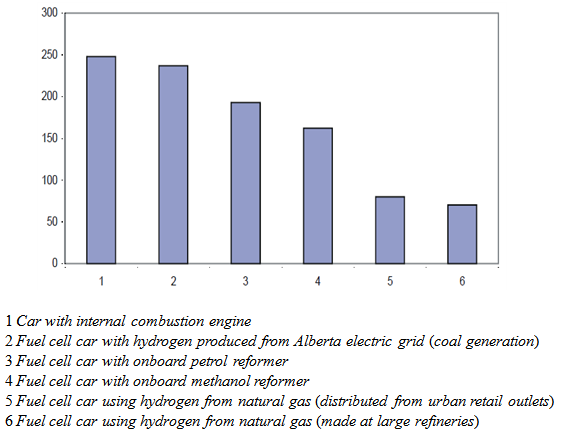 | Figure 4. Graph comparing carbon dioxide emissions (in kilograms per 1000 km) of cars, using different types of fuel sources [32] |
4.6. Direct Dangers in Use and Safety
- In fact, hydrogen has the widest explosive/ignition mix range with air of all the gases and it has been considered relatively as a more dangerous fuel [34]. Additionally, hydrogen flames are difficult to see so may be difficult to fight. Most of these problems are offset in reality by the fact that hydrogen rapidly disperses by lifting off the scene due to buoyancy, and this is true to some extent of hydrogen fires.Figure 5 shows a simulated demonstration of hydrogen and a petrol fire in a vehicle by DaimlerChrysler [1]. The hydrogen fire burns upward, and is unlikely to ignite other parts of the car. The petrol fire, on the other hand, spreads around the entire vehicle and is an obvious danger to anyone in it.
 | Figure 5. A demonstration of a hydrogen and a petrol fire in a vehicle [1] |
5. Conclusions
- Around the globe, interest in hydrogen fuel is growing rapidly, and so is competition to develop the technologies needed to make its widespread use a reality. Funding has come from both private and government sources in the USA and Canada. Even though, there are many problems to be solved before hydrogen can serve as a universal energy medium.Buying these hydrogen vehicles, proffers the best solution, among the available alternative fuels, to reduction or complete elimination of the hazardous vehicle emissions and their environmental effects.It is not possible to predict whether this technology will dominate however, in the future we may have two main fuels as we currently have or perhaps more. The consumer will most likely make the decision as to which fuels will dominate, with the various companies providing the many options. But efforts are currently underway to produce, store and distribute hydrogen in cost effective ways, this shows that there is a great prospect for hydrogen as a fuel in automobiles.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML