-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2014; 4(2): 21-25
doi:10.5923/j.ijee.20140402.01
Moringa Oleifera Oilseed as Viable Feedstock for Biodiesel Production in Northern Nigeria
Abdu Zubairu, Fatima Sani Ibrahim
Department of Chemical Engineering, University of Maiduguri, Borno State, Nigeria
Correspondence to: Abdu Zubairu, Department of Chemical Engineering, University of Maiduguri, Borno State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Biodiesel is an alternative to conventional petro diesel; it is a mono alkyl ester of either vegetable oil or animal fat. In the case of vegetable sources, biodiesel has been produced from numerous oil seeds such as canola oil, palm, and castor oil. In this work biodiesel was produced from moringa oleifera oil seed. The moringa oleifera oil was extracted in the laboratory scale using Soxhlet extractor which is connected to a reflux condenser and a heating mantle at 60℃. After acid pretreatment the oil was transesterified using methanol with sodium hydroxide as catalyst. The methanol to oil molar ratio of 6:1 for transesterification reaction was ensured. The biodiesel synthesized was characterized. The flash point, cloud point, pour point, kinematic viscosity and the acid number of the biodiesel were 135℃, 18℃ and 17℃, 4.85 mm2s-1 and 0.26 mg KOH/g respectively. Comparisons were drawn in line with ASTM D6751 literature standards. Discussions is proffered on the viability of the moringa oleifera oilseed as a feed stock for biodiesel production relative to other oilseeds that are commonly utilized.
Keywords: Moringa Oleifera, Biodiesel, Mono alkyl ester (MAE), Free fatty acids (FFAs), Transesterification
Cite this paper: Abdu Zubairu, Fatima Sani Ibrahim, Moringa Oleifera Oilseed as Viable Feedstock for Biodiesel Production in Northern Nigeria, International Journal of Energy Engineering, Vol. 4 No. 2, 2014, pp. 21-25. doi: 10.5923/j.ijee.20140402.01.
Article Outline
1. Introduction
- Mankind explores various forms of energy in many ways to improve standard of living, this includes better lifestyle, portable water, enhanced agricultural productivity, improved health care and education. Consequently, the world energy consumption is tremendously increasing, which also arguably, is related to the growing global population. The world energy consumption is predicted to increase to around 715 EJ by the year 2030 [1]. The global energy need is primarily obtained from crude oil, coal and natural gas sources. Significant contribution is also derived from hydroelectricity and nuclear. With the exception of the last two, all these supplies are finite in the current usage rates; hence are non-renewable. In addition, the use of these fossil fuels is related to several other drawbacks including increase greenhouse emission and high cost of production. It is therefore imperative to seek for alternative sources of energy to fossil based fuels.Currently, Nigeria imports about 80% of its refined petroleum products [2] which have been hit hard by rapidly increasing cost of crude oil in the world market. In addition, in the Niger Delta region of southern Nigeria where Nigeria’s crude oil is largely produced, there have been cases of severe environmental impacts which have received very little consideration over the years from different administrations and the industry regulators, partly in an effort to fast track development in the oil sector. It is argued that the outcome of decades of neglect has generated the militancy witnessed from the host communities making oil exploration and production activities immensely difficult and risky venture. More also, it has led to series of vandalism of oil installations sometimes with fatal consequences, as well as concomitant loss of oil revenue to the nation. It is worth noting that oil revenue forms the backbone of Nigeria’s economy. Sequel to this instability, and indeed due to similar scenarios as a result of political instability in many other oil producing nations; particularly among many of the member states of the organization of petroleum exporting countries (OPEC), the price of crude oil has continue to rise in the world market. These uncertainties have increased the concern on crude oil based energy security worldwide. Hence in the medium term (2008-2015) and long term (2016-2025), Nigeria envisions an energy transition from crude oil to renewable energy. Among the alternative renewable energy sources are bio fuels obtained from biological sources, and one that have generated enormous interest due to its versatility is biodiesel.
2. Biodiesel Production from Moringa- oleifera Oilseed
2.1. Biodiesel
- Biodiesel is the mono alkyl ester of long chain fatty acids derived from vegetable oil or animal fat [3]. It is a clean renewable fuel that can be sourced from various biomass feedstocks such as waste vegetable oil, yellow grease, animal fats and virgin oils. The fact that biodiesel is derived from vegetable oils and animal fats, potentially, makes it an inexhaustible source of energy, hence renewable. Biodiesel is a fuel with lower rate of emission compared to petro diesel because it emits little or no greenhouse gases on combustion in ignition engines. It is safe to handle because of its relatively high flash point. Furthermore, biodiesel has a low soot emission as it contains little or no sulphur as well as the carcinogenic polyaromatic components. In addition, biodiesel has higher lubricating property than petro diesel thus very promising maintenance potentials to industries that use diesel as fuel. Biodiesel is biodegradable, non-toxic and has low emission profiles as compared to petroleum-based diesel. Overall, the use of biodiesel will allow balance to be sought between agriculture, economic development and the environment. The most common type of feedstock use for biodiesel production are the edible vegetable oils such as soybean oil, rapeseed oil, coconut oil, palm oil and sunflower oil [3]. The food grade vegetable oil known as the refined oil holds the major share as feedstock for biodiesel production because of low free fatty acid and water content [4]. However, the choice of feedstock depends on the availability of oil crops in a particular region. Oilseed crops such as soybean, rapeseed, canola, palm and sunflower are particularly useful for producing biodiesel. Biodiesel production from these sources however, had increasingly placed strain on their prices and availability for use in food production. Therefore, the search for additional regional biodiesel feedstock is an important objective. Some examples may include studies of biodiesel production from less common or unconventional oilseeds such as jatropha, tobacco, moringa oleifera, pongamia pinnata, and rubber oil. In northern Nigeria, one of the most common oil crops is moringa oleifera that can easily be sourced for production of biodiesel both in small and large scale commercialization in the region.
2.2. Moringa oleifera Oilseed
- Moringa oleifera commonly referred to as drum stick tree is also known as ‘okwe oyibo’ in Igbo language, ‘zogale’ in Hausa language and ‘Adagba maloye’ or ‘Ewe igbale’ in Yoruba language [6]. It is a perennial softwood tree of about 5 - 10m in height is popular for its variety of traditional and industrial uses [7]. It is the only genus in the family moringaceae, this genus comprises of 13 species from Africa, Madagascar, Western Asia and Indian subcontinent. Among the 13 species, moringa oleifera is the most widely utilized [5]. Moringa oleifera grows rapidly in most regions and climatic conditions in northern Nigeria [6]. The fast growing drought tolerant moringa oleifera can particularly tolerate the poor soil and the wide range of seasonal rainfall typical of northern Nigeria.When fully mature, moringa oleifera kernel is surrounded by a lightly wooded shell with three papery wings as shown in Figure 1. Moringa oleifera seeds are round or triangular shaped. Figure 2 shows un-de-husked and de-husked moringa oleifera seeds.
 | Figure 1. Mature fruits of moringa oleifera |
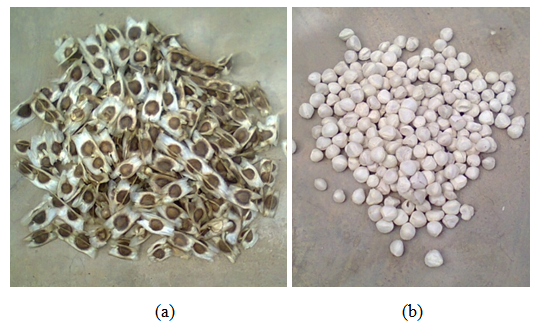 | Figure 1. Moringa oleifera seeds: (a) Un-de-husked (b) De-husked |
2.3. Transesterification Process
- The chemical process by which biodiesel is produced is known as transesterification reaction. It involves a TAG reaction with a short-chain monohydric alcohol normally in the presence of a catalyst at elevated temperature to form fatty acid alkyl esters (FAAE) and glycerol. It is primarily the displacement of alcohol from an ester by another in a process similar to hydrolysis, except that alcohol is used instead of water. The reaction reduces the high viscosity of triglycerides present in vegetable oil or animal fat. Although the most common alcohol used for the production of biodiesel is methanol, other alcohols like ethanol, iso-propanol, propanol and butanol are often used to produce biodiesel. The choice of the alcohol to be used is largely dictated by price; sometime ethanol is cheaper than methanol in some regions. The type of alcohol used also determines the biodiesel grade that is to be produced. For instance, methanol, iso-propanol and propanol are normally produced from petrochemical materials such as methane which is obtained from natural gas [9]. And if the alcohol used for the production of the biodiesel is produce from biological materials, it yields biodiesel that is completely bio-based. Equation 1 illustrates the general form of transesterification reaction.
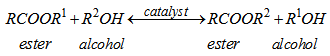 | (1) |
3. Methodology
- Moringa oleifera seeds were collected domestically from University of Maiduguri campus premises and allowed for for some days to dry. More of the seeds were purchased from Maiduguri Monday market. Other chemicals and reagents (methanol, n-hexane, and sodium hydroxide) were commercial grades manufactured by Aldrich chemical company were purchased at local stores.
3.1. Extraction of Moringa oleifera Oil
- The moringa oleifera dried seeds of total weight 322.86 g were first de-husked after which they were crushed into powder form by the use of mortar and pestle to allow for easy solvent contact and better extraction. Various factors such as the type of solvent, solid: solvent ratio and extraction time affect oil extraction efficiency depending on the kind of oil seed. In this work n-hexane was used as the solvent and a solid:solvent ration of 1:6 w/v ratio and extraction time of 6 h was in line with previous recommendation [10]. The powdered form of the seeds was then fed to Soxhlet extractor containing n-hexane; the system was connected to a 2 litres round bottom flask and a reflux condenser. The extraction was carried out for 6 h after which the solvent was evaporated over a water bath until completely recovered. The acid value of crude moringa oleifera oil was determined as 3.2 necessitating acid pre-treatment before transesterification.
3.2. Transesterification Reaction
- The transesterification of moringa oleifera oil was carried out using methanol. The process was conducted according to standard procedure with 6:1 molar ratio of methanol to moringa oleifera oil for period of 1 h at 60℃. A 5 % by weight NaOH was used as the catalyst. Lower catalyst concentration of up to 1% have been reported in the literature for biodiesel synthesis from moringa oleifera. As illustrated in [10] the higher the catalyst concentration the higher the biodiesel yield. A fresh solution of methanol and sodium hydroxide was prepared to maximize catalyst usage. The batch was set up by charging and preheating the oil in the reactor, and an established amount of the catalyst-methanol solution was then added and the reaction was timed. At the end of the reaction the mixture was allowed to cool to room temperature without agitation and settle to two phase separation under gravity. The upper phase of the mixture was moringa oleifera methyl ester (MOME) i.e. the biodiesel while the lower phase consist of glycerol, excess methanol, the catalyst, soap formed during the reaction, some entrained MOME and traces of glycerides. The two phases where separated by decantation. The MOME was purified by gently washing with distilled water until a desired purity was achieved.
3.3. Determination of Properties of Oil and Biodiesel
- The crude moringa oleifera crude oil and the synthesized biodiesel properties were determined following the ASTM D6751 and EN 14214 standard specifications. The flash point, cloud point, pour point, kinematic viscosity and the acid value for the biodiesel were determined following the ASTM standards D93, D2500, D97, D445 and D664 respectively.
4. Results and Discussion
4.1. Moringa oleifera Oil Yield
- The oil from moringa oleifera seed was extracted with n-hexane as solvent in the Soxhlet extractor. The extracted oil was pale yellow liquid at ambient temperature with characteristic unique odour. The oil yield of moringa oleifera was determined as 34.4% w/w which is in agreement with previous literature [5].
4.2. Iodine Value of Moringa oleifera Oil
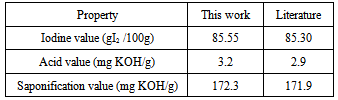
- The extracted moringa oleifera oil was analyzed to determine the iodine value. The iodine value was obtained as 85.55 I2 100 g, this value is in close agreement with that of previous literature [6] as shown in Table 1. The low iodine value of this oil makes it suitable for biodiesel production since high iodine value leads to the formation of deposits on engines and problem during storage of the fuel.
4.3. Acid Value Moringa oleifera Oil
- Acid value of oil is an important parameter which affects the transesterification of oils. Oils which have high acid values will produce soap during transesterification. The extracted oil was analyzed to determine its acid value, the acid value was found as 3.2 mg KOH/g which agrees well with previous literature [6] as shown in Table 1. The high acid value of moringa oleifera oil necessitated acid pretreatment of the oil before transesterification.
4.4. Saponification Value Moringa oleifera Oil
- The saponification value of moringa oleifera oil was determined as 172.3 mg of soap (as potassium oleate)/g of oil which is in agreement with previous literature [6] as shown in Table 1.
|
4.5. Properties of the Moringa oleifera Methyl Ester (MOME)
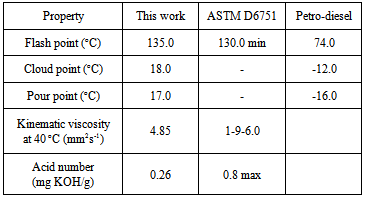
- The properties of the biodiesel produced were analysed. Properties tested were flash point, cloud point, pour point, kinematic viscosity and the acid number. The values of all properties determined agreed well with the recommended standards for biodiesel. Table 2 shows the properties of moringa oleifera methyl ester in comparison with the standard and that of conventional petroleum diesel.The flash point is a measure of flammability classification of fuels. Typically, pure methyl esthers were reported to have flash point in the range of 200℃ and above [11], thus making them to be strongly non-flammable relative to conventional petro-diesel. However, in the course of biodiesel synthesis and purification not all the methanol used is converted in to biodiesel or recovered from the biodiesl hence the flammability limit drops significantly. Excess methanol may also corrode engine seals and metallic machine components when run on biodiesel. As may be seen from Table 2, the flash point of moringa oleifera methyl ester was found to be 135℃ and the ASTM standard stipulates that biodiesel may be dangerous to handle if the flash point falls below 130℃. The cloud point is the temperature at which a cloud of wax crystal first appear in the biofuel sample when it’s cooled. It is significant to determine the cloud of the biodiesel as higher values of these properties indicates that biodiesel will form gel at higher temperatures which is highly likely to cause engine damage. This parameter is critical for cold weather regions in biodiesel performance engines. In this work, the cloud point was found to be 18℃ which indicates that moringa oleifera biodiesel may not be suitable for very cold weather condition without modification by blending with petro-diesel. The pour point on the other hand is the temperature at which the fuel can no longer be freely poured. The pour point for moringa oleifera biodiesel was determined as 17℃ which agree well with that of previous literature [5].The kinematic viscosity of the biodiesel which may be viewed as “the resistance of the biodiesel to flow under gravity”. The kinematic viscosity of the moringa oleifera biodiesel was determined as 4.85 mm2s-1 according to the ASTM D445 standard which agrees well with the reported literature [5, 12]. Is worth noting that the fundamental difference between the chemical nature of transesterificated and untransesterificated biofuels is in their viscosity values. Arguably, the major aim of producing alkyl-esters from vegetable oils and animal fats containing TAGs is the significant reduction in their viscosities [11]. The high viscosity values of the oils leads to operational problems in diesel engines.
|
5. Conclusions
- Maringa oleifera oil was extracted from moringa oleifera oilseed and fatty acid methyl ester of the oil (biodiesel) was successfully produced using an alkali catalyzed transesterification reaction with methanol, after acid pretreatment of the crude moringa oleifera oil. The oil yield from moringa oleifera seeds was 34.4% by weight. The moringa oleifera oil was characterized; the results showed that the oil has an acid value of 3.2 mg KOH/g, an iodine value of 85.55 g I2 /100g and a saponification value of 172.3 mg KOH/g. The biodiesel properties; flash point, cloud point, pour point, kinematic viscosity and acid number were also determined. The moringa oleifera methyl ester was found to have a flash point of 135℃ which agrees well with previous literature. The high flash point was due to the excess alcohol present in the biodiesel which was not removed during washing. While the cloud point and pour point of moringa oleifera methyl ester were found to be 18℃ and 17℃ respectively. The kinematic viscosity is 4.85 mm2s-1 and acid number was 0.26 mg KOH/g.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML