Gautam Kumar Roy
Quality Control Department, Indian Oil Corporation Ltd., Barauni Refinery, Begusarai, 851117, India
Correspondence to: Gautam Kumar Roy, Quality Control Department, Indian Oil Corporation Ltd., Barauni Refinery, Begusarai, 851117, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The increasing demand of energy sources is the cause of high price of hydrocarbon products. This has led to a driving force for converting polyethylene into useful hydrocarbon products. More than 80 million barrels of petroleum is being consumed per day worldwide and out of 195 countries only 40 countries produce petroleum. Rest fulfill there requirements by importing them. Emission of harmful gases such as NOx, SOx, CO, CO2 and other Greenhouse gases contributes to the global warming and also causes enviournmental and health problems. Waste plastic can cause many enviournmental and health related problems when it is land filled as it is not biodegradable and remain in landfills for hundred of years[3]. Waste plastic depolymerisation process can be carried out by hydropyrolysis process in which the solid waste plastic is converted into gases, gasoline, middle distillate and heavy oil products. Polyethylene can be converted into alkanes and alkene products by thermal degradation process in which the depolymerisation of higher hydrocarbons such as C40+ components can be cracked into lower hydrocarbons. This paper describes the low temperature hydropyrolysis of polyethylene into hydrocarbon product and its fractionates. The products derived, there characteristic properties, enviournmental and economic feasibility were also studied.
Keywords:
Fuel, Hydrocarbon, Plastroleum, Waste Polyethylene, Hydropyrolysis, Gas, Naphtha, Middle Distillate, Heavy Oil
Cite this paper: Gautam Kumar Roy, A Novel Process of Converting Polyethylene to Gasoline, Middle Distillate and Heavy Oil through Hydropyrolysis Route, International Journal of Energy Engineering, Vol. 3 No. 3, 2013, pp. 147-157. doi: 10.5923/j.ijee.20130303.04.
1. Introduction
Waste plastic represents a large amount of municipal waste. New technologies are being developed that allow more material to be recovered and new value created from those materials. Solid waste management continues to evolve and much of the evolution is driven by the adoption of new technologies that increases recovery capacity and processing capacity[1]. Plastic, a non biodegradable materials which are generally made up of variety of organic resins , most popular of which are high density polyethylenes (HDPE), low density polyethylenes (LDPE) and polyethylene terephthalates etc.. These are resins used for making different types of plastic bottles of water and soft drinks, polybags, etc. These polymers are made up up of long chain carbon atom and hydrogen atom(-CH-)n along with oxygen , sulfur and/or nitrogen. These are part of the chain linking in which large number of repeated units of monomers(-CH-) binds together. Plastics generally degrade very slowly, because of the chemical bond that make it durable and resistant to natural activities. 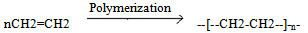 There are two types of plastic, Thermoplastic and Thermosetting plastic. Thermo plastic are the plastic that do not undergo chemical change in there composition when heated, some examples are polyethylene, polypropylene, polystyrene etc. Thermosetting plastics melts and take shape ones. Chemical reaction in thermosetting plastics are irreversible.Plastic to oil technology is a two fold transformation of non-recyclable plastic into useful commodities and creating a reliable source for an alternative energy from an abundant at no extra cost for feedstock. The conversion from polyethylene to oil is a depolymerisation process to convert scrap polyethylene into petroleum products. This process is utilized to convert long chain polymers to monomers that can be used to rebuild resins having the property and performance of virgin resins.In this paper the author have described the process of converting scrap polyethylene preferably low density polyethylene(LDPE) into useful gaseous and liquid hydrocarbon products (gasoline and middle distillate) through hydropyrolysis route. Pyrolysis is the thermochemical decomposition of organic material at elevated temperature in the absence of oxygen[2] .In hydropyrolysis process the pyrolysis process is carried out in the presence of water. The product obtained plastroleum (plastic+petroleum (derived from plastic)) through hydropyrolysis process have some distinct properties. The conversion of polyethylene generally low density polyethylene(LDPE) into useful hydrocarbon products will change the enviournmental concerns of soil and water pollutions due to landfills and sewage choking problems. This process also help in reducing the greenhouse gases in the enviournment such as NOx, SOx, CO2, CO etc. The aim of this research is to study the characteristic property of the products obtained by the low to medium high temperature hydropyrolysis of plastroleum products obtained from polyethylene. The enviournmental and economic feasibility of the products were also discussed in this paper. The depolymerisation and repolymerisation reaction carried out in the process were also studied. The chromatographic study of gases and the liquid hydrocarbon (naphtha) and the FTIR (Fourier Transform Infra Red Spectroscopy) of the plastroleum crude, naphtha and the middle distillate samples were also studied. The water in which the plastroleum is recovered is also analysed and the total oil content in it, is determined.
There are two types of plastic, Thermoplastic and Thermosetting plastic. Thermo plastic are the plastic that do not undergo chemical change in there composition when heated, some examples are polyethylene, polypropylene, polystyrene etc. Thermosetting plastics melts and take shape ones. Chemical reaction in thermosetting plastics are irreversible.Plastic to oil technology is a two fold transformation of non-recyclable plastic into useful commodities and creating a reliable source for an alternative energy from an abundant at no extra cost for feedstock. The conversion from polyethylene to oil is a depolymerisation process to convert scrap polyethylene into petroleum products. This process is utilized to convert long chain polymers to monomers that can be used to rebuild resins having the property and performance of virgin resins.In this paper the author have described the process of converting scrap polyethylene preferably low density polyethylene(LDPE) into useful gaseous and liquid hydrocarbon products (gasoline and middle distillate) through hydropyrolysis route. Pyrolysis is the thermochemical decomposition of organic material at elevated temperature in the absence of oxygen[2] .In hydropyrolysis process the pyrolysis process is carried out in the presence of water. The product obtained plastroleum (plastic+petroleum (derived from plastic)) through hydropyrolysis process have some distinct properties. The conversion of polyethylene generally low density polyethylene(LDPE) into useful hydrocarbon products will change the enviournmental concerns of soil and water pollutions due to landfills and sewage choking problems. This process also help in reducing the greenhouse gases in the enviournment such as NOx, SOx, CO2, CO etc. The aim of this research is to study the characteristic property of the products obtained by the low to medium high temperature hydropyrolysis of plastroleum products obtained from polyethylene. The enviournmental and economic feasibility of the products were also discussed in this paper. The depolymerisation and repolymerisation reaction carried out in the process were also studied. The chromatographic study of gases and the liquid hydrocarbon (naphtha) and the FTIR (Fourier Transform Infra Red Spectroscopy) of the plastroleum crude, naphtha and the middle distillate samples were also studied. The water in which the plastroleum is recovered is also analysed and the total oil content in it, is determined.
2. Experimentation and Methodology
Waste polyethylene material used for this process are LDPE and HDPE type of polyethylene materials.The polymerisation reaction of Polyethylene materials generally follow three step process for there combination into a large polymer materials. These steps areInitiation –In this step the chain is initiated by the hemolytic fission of the radical | (2) |
Chain Propagation step –in this step the the free radical produced in the above step combines with an alkene molecule to produce new free radical | (3) |
 | (4) |
 | (5) |
And the final step is chain termination stepThis process of chain reaction is terminated by combination or disproportion that is combination or loss of anion. | (6) |
 | (7) |
The termination of the process results in the dead polymer or unreacted polymer[15]. The depolymerisation of the polyethylene material occurs by the cleavage of the long chain ethylene molecule into free radical components by thermal degradation. The free radical on condensation combines with the released hydrogen atom to terminate into shorter and medium range chain structure. These short chain molecules generally contains the hydrocarbon components ranging from C1 to C40. The depolymerisation reaction carried out have three steps.Initiation of Depolymerisation a) Random chain scission  | (8) |
b) Chain end initiation | (9) |
Depropagation  | (10) |
Chain transfer involving random chain scission | (11) |
c) Termination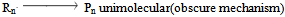 | (12) |
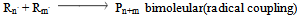 | (13) |
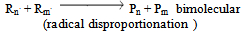 | (14) |
Where P, R. , M denotes polymer radical and monomer respectively and subscripts chain and degree of polymerisation[5].In this paper the study of low temperature thermal degradation of the polyethylene material is studied. The product plastroleum crude developed by hydropyrolysis of waste polyethylene at low temperature range from 70℃ to 395℃ . The residual gases from C1 to C5 range is also studied. Different physical properties of the plastroleum crude and the fractionate of the plastroleum were also discussed. The experimentation of the process is carried out on a lab scale and the different aspects of such process were studied.The waste polyethylene materials were first collected (generally LDPE and HDPE types of polyethylene materials) and were washed and then dried .Then these waste polyethylene materials were cut into small Pilates or small pieces.( Figure 1 shows the schematic flow diagram of the hydropyrolysis process to produce plastroleum crude and the different fractionate products recovered from such process). The waste plastics were again dried using hot air to remove moisture (if any). Then small waste plastic pieces were inserted in a vessel 1 or reactor through a hopper as shown in Figure 1. One end of the vessel or reactor is connected with a recovery vessel 2 containing de-mineralized water having temperature 8℃ to 25℃ through a connecting pipe. The temperature of the vessel 2 is maintained by putting it into a low temperature water bath. The vessel 2 has one opening for the gases to be collected in another vessel 3. The vessel 3 is placed in a chilled water bath so that the lighter hydrocarbon (if any) get condensed as liquid. Another end of the vessel 1 or reactor is connected with temperature probe or thermometer.The hydropyrolysis process was started by heating the vessel 1 at different temperature ranges. As soon as the heating starts, the air or any residual gases escapes through the receiver vessel 2 and 3. As the heating continues starting from lower temperature range the gasification process of polyethylene starts, from temperature range 70℃- 100℃, the polyethylene material starts melting as the temperature increases further gradually. The gases so formed from such gasification process, passes through the pipe and recovered through water in vessel 2. The gases get condensed in water having temperature 8℃ to 25℃. The uncondensed gases having carbon number C1 to C4 and C5 escapes from the water and comes out and collected in separate connected vessel 3. The vessel 3 also contains chilled water so that any residual uncondensed gas escapes out from vessel 2 get condensed in vessel 3. The remaining gases which did not get condensed in Vessel 3 escapes out and collected in a separate gas collector vessel. The condensed gases floats over the water as liquid product in vessel 2 and vessel 3. This indicates lighter density of plastroleum product than water. The temperature was increased gradually. It was found that maximum recovery of plastroleum products is observed at temperature 120℃ to 160℃. As the temperature is increased the heavier oil were recovered and wax formation starts in water. The maximum temperature reached is about 395℃. The total time took to recover the plastroleum from polyethylene generally LDPE and HDPE is about 210 - 350 minutes . Primary decomposition of the polyethylene waste materials generally produces 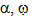 olefin,
olefin,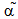 olefin and n-paraffin constituents. Polyaromatic components are produced on heating the polyethylene material at higher temperature[4] . At lower temperature the generation of lighter gaseous hydrocarbon product increases range from C1 to C5. The low temperature hydropyrolysis of polyethylene material enhances the production of plastroleum crude and hydrocarbon gases with. Prolonged heating of polyethylene material for longer time at specified controlled temperature helps the longer hydrocarbon chain to crack. Depolymerisation and repolymerisation of the cracked product into shorter chain and branched chain hydrocarbon products also increases the olefinic content. The increase in the olefin content and iso-paraffinic components increases the antiknocking properties of the product. The aromatic content increases with time and temperature which is due to the reaction of ethane and propene. It was observed that with higher temperature and longer pyrolysis time the heavier products decomposes to lighter one, because at higher pyrolysis time the carbon chain was exposed to more forceful thermal decomposition effect[11] . The residue material which is left in the bottom of the vessel contains, generally a black char material having components which are used as binders or additives and contaminants which enters in the waste polyethylene material as part of feedstock. These are generally residue containing calcium carbonates, sludge, heavy materials, metals, clay and carbon black. The above flow diagram shown in Figure 1 gives idea about the process of extracting plastroleum crude, gases, naphtha, middle distillate and heavy cut oil( in solid condensed form) (from left to right).
olefin and n-paraffin constituents. Polyaromatic components are produced on heating the polyethylene material at higher temperature[4] . At lower temperature the generation of lighter gaseous hydrocarbon product increases range from C1 to C5. The low temperature hydropyrolysis of polyethylene material enhances the production of plastroleum crude and hydrocarbon gases with. Prolonged heating of polyethylene material for longer time at specified controlled temperature helps the longer hydrocarbon chain to crack. Depolymerisation and repolymerisation of the cracked product into shorter chain and branched chain hydrocarbon products also increases the olefinic content. The increase in the olefin content and iso-paraffinic components increases the antiknocking properties of the product. The aromatic content increases with time and temperature which is due to the reaction of ethane and propene. It was observed that with higher temperature and longer pyrolysis time the heavier products decomposes to lighter one, because at higher pyrolysis time the carbon chain was exposed to more forceful thermal decomposition effect[11] . The residue material which is left in the bottom of the vessel contains, generally a black char material having components which are used as binders or additives and contaminants which enters in the waste polyethylene material as part of feedstock. These are generally residue containing calcium carbonates, sludge, heavy materials, metals, clay and carbon black. The above flow diagram shown in Figure 1 gives idea about the process of extracting plastroleum crude, gases, naphtha, middle distillate and heavy cut oil( in solid condensed form) (from left to right). | Figure 1. Flow Diagram of hydropyrolysis Process of extracting Plastroleum crude and its Fractionate |
3. Analytical Study and Results
The low temperature hydropyrolysis of polyethylene is carried out with temperature range 70℃ to 395℃. More than 90% of the hydrocarbon products in gaseous and liquid form is derived from such process(Figure 2). The amount of product obtained includes the plastroleum crude containing liquid products and heavy cut oil( including waxy products) and the hydrocarbon gases range from C1 to C5 . | Figure 2. Plastroleum , Naptha, Middle Distillate and Heavy Cut oil Derived from Hydropyrolysis process |
The analytical study of the plastroleum crude is carried out, some of the physical properties of the product is described below. The C/H value, calorific value, net calorific value ,molecular ratio are calculated values. The sulfur content, and Density were observed by following ASTM D4294 and ASTM D1298 methods respectively[8],[10].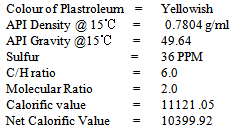
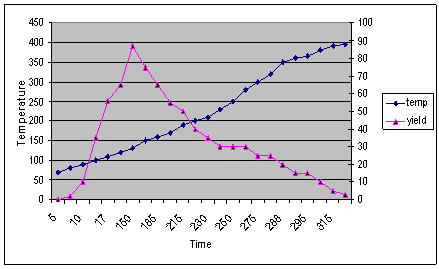 | Figure 3. Depicts the yield of gaseous and liquid hydrocarbon products at different temperature and time |
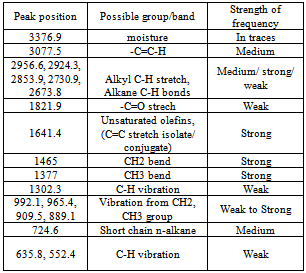
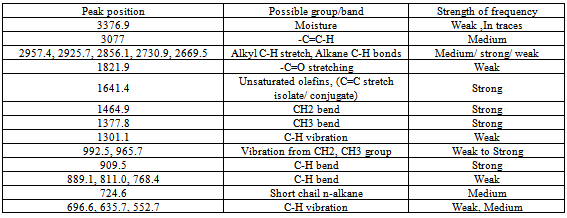
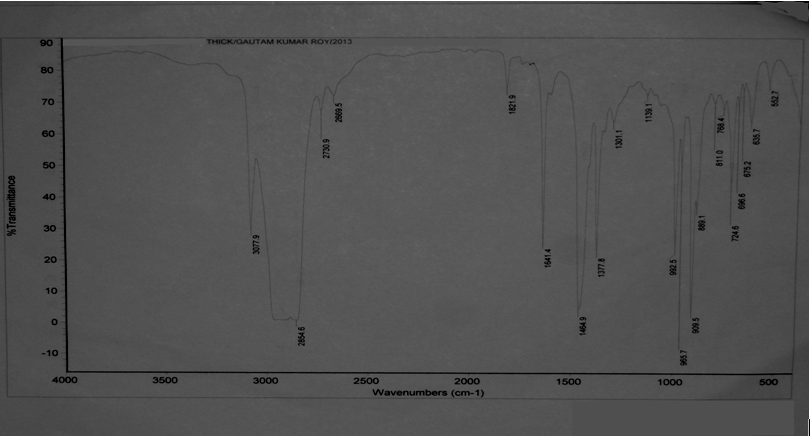
The temperature, yield and time graph of the conversion process of polyethylene into plastroleum product and gases shows that by prolonged heating of the polyethylene material at certain temperature gives maximum yield (Figure 3). As the temperature increases gradually the gaseous product starts forming. The yield of product decreases at elevated temperature because of the presence of bottom residue. The temperature, time and yield graph describes the amount of samples recovered at different elevated temperatures. With prolonged time and temperature the higher yield of gaseous and liquid hydrocarbon products were observed.The FTIR study of the plastroleum crude is carried out and the different functional groups at different frequencies were observed. The thin film and thick film FTIR spectra of the product were analysed for strong and weak band identification (Figure 4, Figure 5 and Table 1). Table 1. FTIR study of plastroleum crude derived from waste polyethylene (LDPE and HDPE)
 |
| |
|
The fractionation of the plastroleum crude was carried out. In this process the plastroleum crude is heated in a flask following ASTM D86 and the different temperature cut of the samples such as naphtha , middle distillate and heavy cut oil were taken out[6]. The naphtha component is extracted up to temperature 1555℃. These samples were collected separately. The gaseous part which is derived during the process of extraction of plastroleum crude from polyethylene is passed through chilled water. The Gas which did not get condensed in the chilled water in vessel 3 (Figure 1), escapes out from it and collected in the gas collector vessel. Gas chromatographic analysis of the residual collected gas was carried out and the different components were observed. The gas components generally contains hydrogen and C1 to C5 components[9],[14]. The different components of the collected gases is described in chromatogram (Figure 6) and (Table 2). 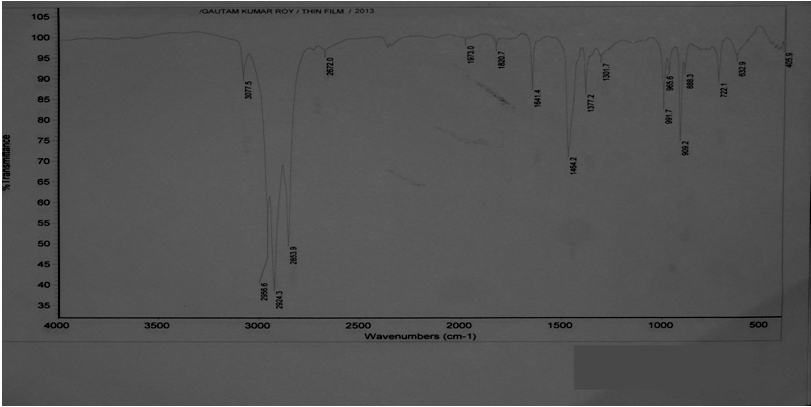 | Figure 4. Describes the thin film FTIR spectra of Plastroleum crude |
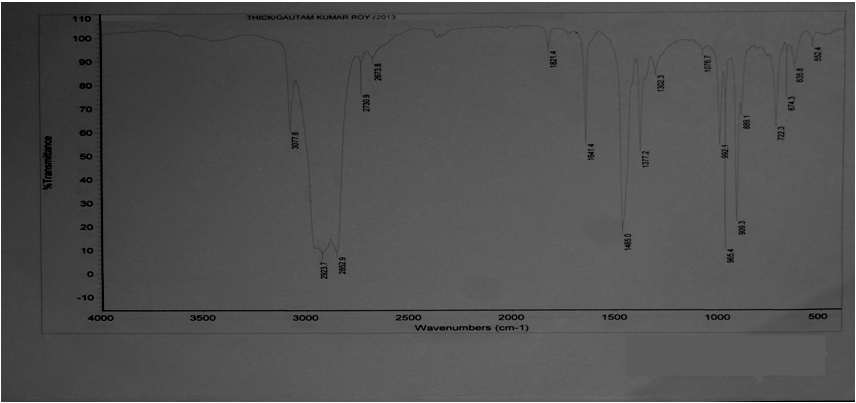 | Figure 5. Describes the thick film FTIR spectra of Plastroleum crude |
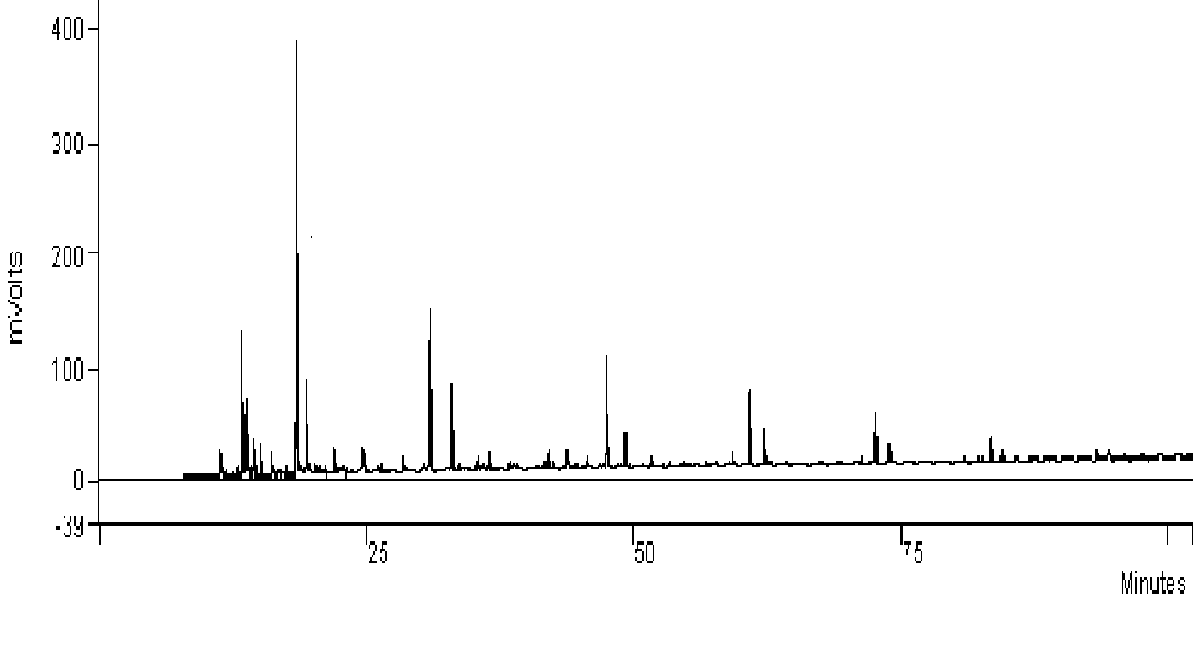
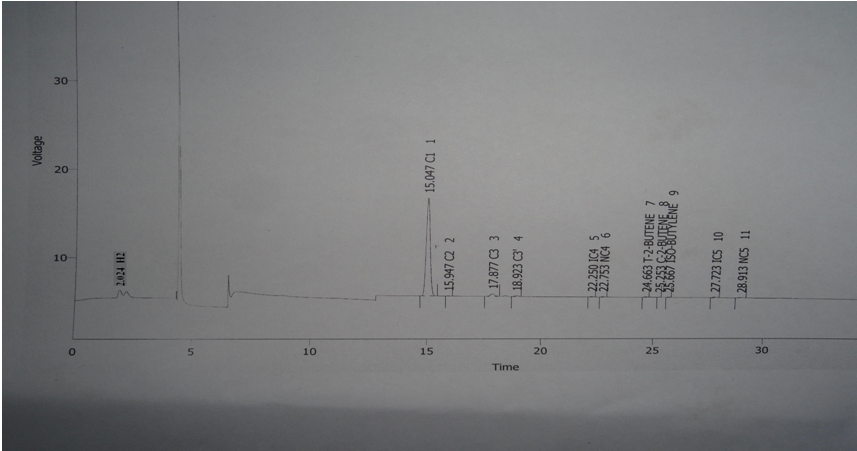 | Figure 6. Chromatogram shows different components of the gaseous hydrocarbon which remains uncondensed after chilling |
The different components of the residual gas contains Table 2. Shows different hydrocarbon components present in gaseous product obtained during hydropyrolysis process
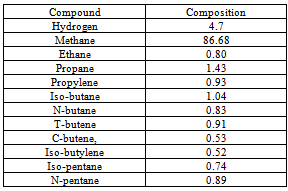 |
| |
|
The residual part left in the flask that is the residue left after the extraction of plastroleum can be used by mixing it with bitumen in road construction, roofing of terraces etc. The fractionation of the plastroleum crude was carried out and the different temperature cut of the samples such as naphtha, middle distillate and heavy cut oil were taken out(Table 3). The different components of the plastroleum crude extracted with temperature cut are Table 3. Shows different temperature cut fractions of plastroleum crude
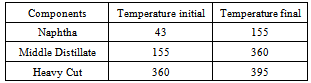 |
| |
|
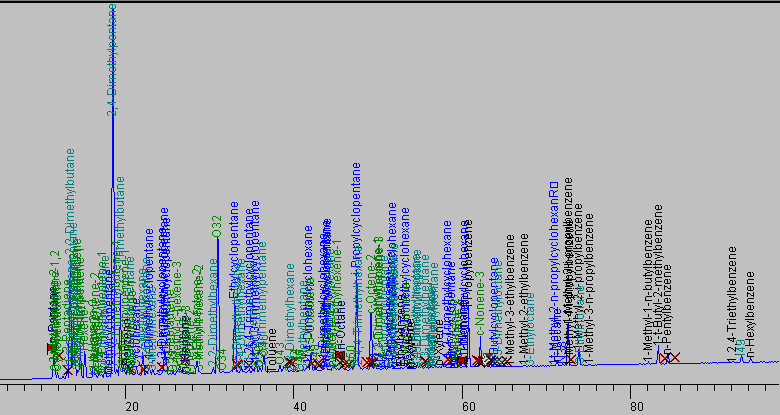
The naphtha components derived from the plastroleum crude contains different hydrocarbon components which are confirmed by the PIONA (Paraffin, Iso-paraffin, olefin, naphthene, aromatic) analysis. The chromatographic study shows components ranging from paraffinic, iso-paraffinic, aromatic, olefinic, naphthenes and some polyaromatic components. The naphtha sample has specific gravity of 0.725 g/ml, and C/H ratio of 5.873 with very slight yellowish colouration. The PIONA analysis of the naphtha sample gives detail idea of the components present in the product. The PIONA analysis of naphtha sample derived from plastroleum crude give more than 150 different hydrocarbon components. Figure 7 and Figure 8 shows the different components present in the naphtha sample derived from plastroleum[7]. The chromatographic study reveals that the naphtha sample contains C5 to C13 components with C7 components in higher concentration in near about 50%. The concentration of different components are given in Table 4.Table 4. components present in naphtha derived from plastroleum crude
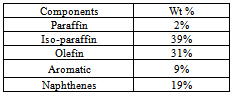 |
| |
|
The paraffinic components present ranging from C5 to C10 that is n-Pentane to n-Decane, olefinic components ranging from C5 to C9 that is from components 2 methyl butene to nonene-3. The aromatics components having concentration of 9% contains C6 to C12 components comprising different aromatic groups with benzene concentration of 0.3%. Iso-paraffinic group components contains C6 to C13 and the Naphthenic group with 19% having components concentration ranging from C6 to C10. The FTIR study of naphtha sample is carried out in thin film and thick film (Figure 9, Figure10 and Table 5). The thin film and thick film study of the sample is carried out to obtain more precise results of the spectra graph. The thin film and thick film FTIR study helps in observing the functional groups in better way.Table 5. FTIR study of naphtha sample derived from plastroleum crude
 |
| |
|
 | Figure 7. Describes the PIONA analysis of the naphtha sample derived from plastroleum crude |
 | Figure 8. Shows component wise PIONA results of the naphtha sample |
 | Figure 9. Thin film FTIR spectra study of naphtha sample |
 | Figure 10. Thick film FTIR spectra study of naphtha samples |
The middle distillate obtained from the plastroleum crude has the boiling range from 155℃ to 360℃. The FTIR study of the sample were also carried out in thin film and thick film for identification of strong and weak bands (Figure 11, Figure 12 and Table 6.)The study reveals the presence of the different functional groups.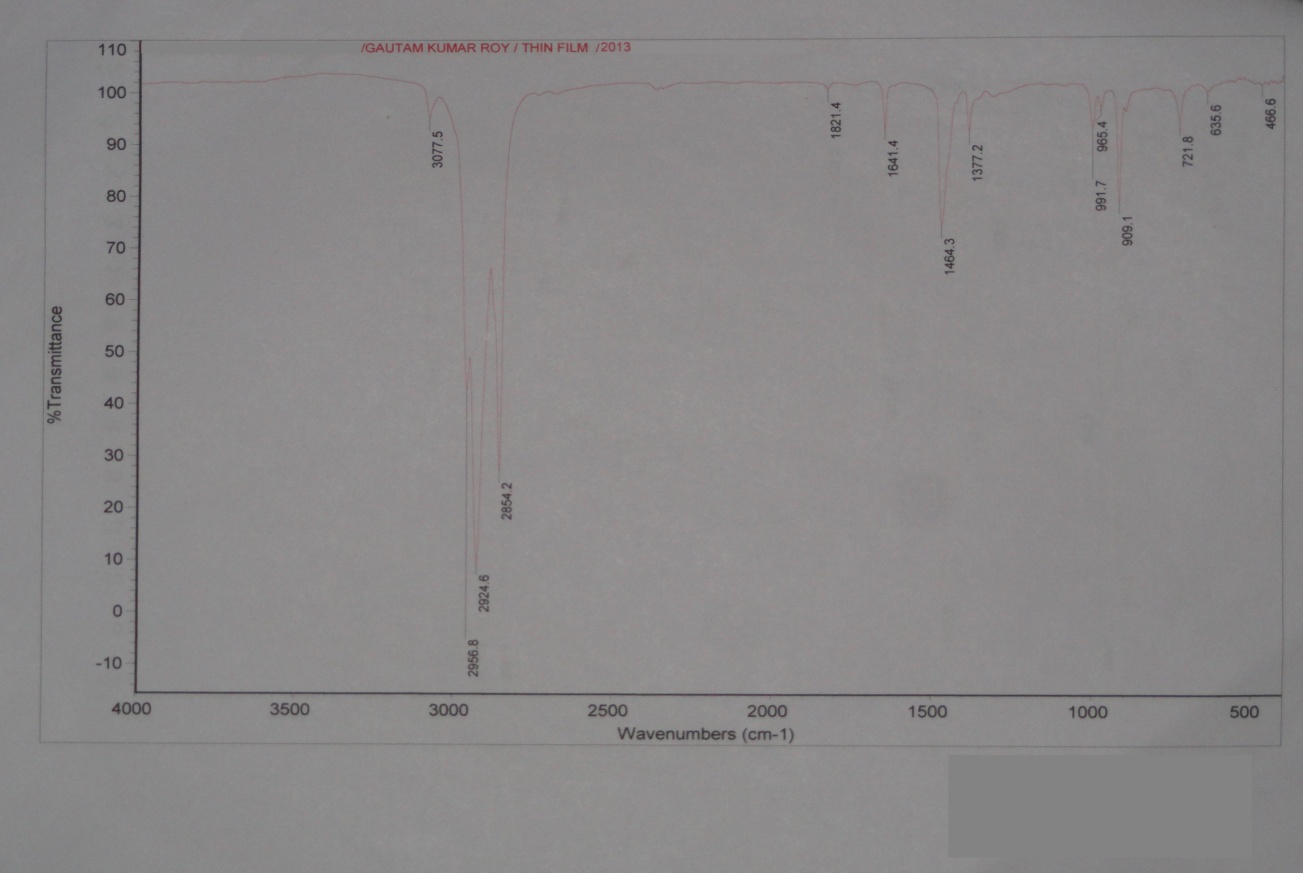 | Figure 11. Thin film FTIR spectra study of Middle Distillate |
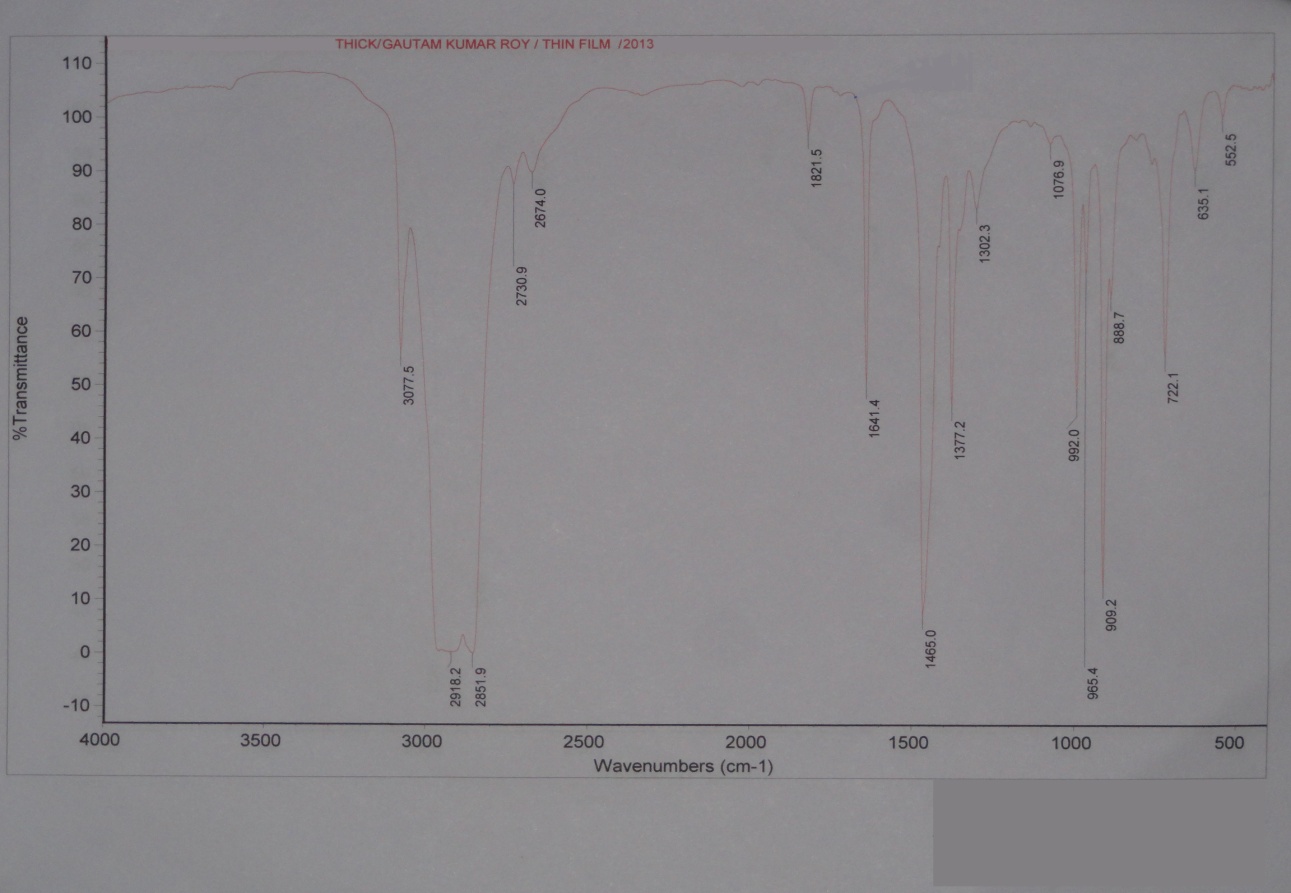 | Figure 12. Thick film FTIR spectra study of Middle Distillate |
The Middle distillate obtained from the plastroleum crude were analysed and it was found that the components are well in the range of Kerosene, ATF and Diesel. The middle distillate component is further fractionated to recover the Kerosene and Diesel components. Maximum percentage of middle distillate fraction is obtained by the fractionation of plastroleum, which is about 68-70%. These components can be used directly with some basic processing or can be blended with the diesel and kerosene oil. The above FTIR study (Figure 10, Figure 11 and Table 6) of middle distillate shows the presence of different functional groups in the extract.Table 6. FTIR study of Middle Distillate sample derived from plastroleum crude
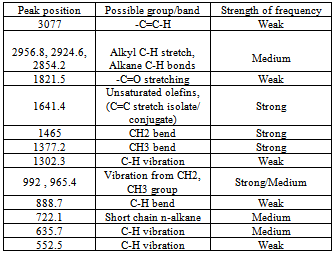 |
| |
|
The property of the middle distillate fraction, found is at par with the conventional middle distillate fractions. The water through which the oil is recovered is analysed and the oil content in it is determined. The oil content in the water is found in the range of 0.9% to 1%. This can be recovered by oil filtering process of water.
3.1. Enviournmental and Economic Feasibility
The economic and enviournmental feasibility of the product is also studied. Hydrocarbon derived from plastic has shown potential to overcome this issue the world faces today although, waste plastic can cause variety of enviournmental and health related problems when solely land filled[3]. This pollution is caused due to the excess amount of waste being thrown away causing the chocking of river streams, drains, and infertility of soil. The use of waste polyethylene into useful hydrocarbon products will help curbing this problem and hence help in making more cleaner enviournment . On economic front this process of waste polyethylene utilization will provide triple profit for any nation, first by producing and selling polyethylene as polybags, bottles etc. and then after using this waste polyethylene, can again be converted into useful hydrocarbon products and sold as gases, gasoline, middle distillate etc. On the third front it will also help in reducing waste caused due to polyethylene materials and help minimizing health related problems and enviournmental pollution.The present global, average per capita consumption of polyethylene is about 26 Kgs and the consumption growth rate is about 5% and it is expected to touch a figure of 227 million tons by 2015. The global polyethylene demand is expected to grow with an average of 4.4 % by 2020[16]. The increase in the production of polyethylene materials will also lead to the increase in solid waste materials. Thus process of recycling these polyethylene materials into useful hydrocarbon products will reduce the solid waste in the enviournment. Also blending of such hydrocarbon products with conventional hydrocarbon products such gasoline, diesel etc. will also help in preserving fossil fuels for our future generation. World wide Oil refining companies will get benefitted by addition of hydrocarbon products from waste polyethylene into conventional gasoline and middle distillate. This will help in reducing there operating cost on crude processing and catalyst use.
4. Conclusions
The hydropyrolysis of plastroleum crude is carried out at low temperature rang from 70℃ to 395℃. Prolonged heating of polyethylene at certain temperature enhances gasification and oil recovery which are in comparison with the commercial naphtha and middle distillate. The experiment carried out in the lab scale produces more than 90 % recovery of gaseous and liquid hydrocarbon products. Low temperature prolonged heating of polyethylene materials produces higher recovery of oil than heavy waxy materials. The percentage of gaseous and liquid products increases with temperature and time. The physical properties of plastroleum crude compared favorably with the petroleum products. At certain optimum temperature maximum amount of gaseous and oil recovery is observed. The products can be used directly with certain modification or can be blended with the conventional petroleum products for higher production of hydrocarbon products. The study is generally carried out with low density polyethylene (LDPE) and High density polyethylene (HDPE) materials, the waste utilization, enviournmental concerns and economic feasibility were also studied.
ACKNOLEDGEMENTS
The Author is indebted to Sri S. K. Jha , Executive Director, Barauni Refinery, Indian Oil Corporation Limited, Sri R. K. Chugh , General Manager, Technical Services , Barauni Refinery, Indian Oil corporation Limited, Sri S. N. Pandey, DGM Technical Services, Barauni Refinery, Indian Oil Corporation Limited, Mr. A..S. Sarkar, Deputy Manager , Quality Control Laboratory, Barauni Refinery, Indian Oil Corporation Limited for there encouragement and support. The author is also grateful to Dr. Atul Ranjan Scientist, RPBD, and Mr. G. M. Bahuguna Senior Technical Officer, Indian Institute of Petroleum (IIP), Deheradun and all other associated Scientists of Indian Institute of Petroleum (IIP) Deheradun for extending there helping hand and support in analyzing the products. The author is also grateful to Mr. Ashok Kumar Roy, Assistant General Manager, Metal Junction Private Limited, Rourkela for providing guidance and support to complete this paper.
References
| [1] | 4R sustainability inc. Portland Conversion molecular ratio technology: A compliment to recycling technology , April 2001, 3,6 |
| [2] | C.O. Osueke, O.I. Ofondu, Conversion of waste plastics (polyethylene) to fuel by means of pyrolysis, International Journal of Advance Engineering Sciences and Technologies Vol. No. 4, Issue No. 1, 21 |
| [3] | Moinuddin Sarker, Mohammad Rashid and Mohammad Molla, Waste plastic converting into hydrocarbon fuel materials, Dept. of Research and Development, Natural state Research Inc.,1 |
| [4] | Ademiluyi T, Akpan C, Preliminary Evolution of Fuel oil produced from pyrolysis of low density polyethylene water-sachet wastes, Journal of applied science and enviournment management, vol 11 (3) 15-19, Para 2 , 15 |
| [5] | Premamoy Ghosh, Polymer Science and Technology, polymer characteristics and polymer characterization, 210-211, 6.4.2 |
| [6] | ASTM D86-09, Standard Test Method for Distillation of Petroleum Products at Atmospheric Pressure,1-27 |
| [7] | ASTM D5134 -08, Standard Test Method for Detailed analysis of Petroleum Naphthas through n-Nonane by Capillary Gas Chromatography, 1-11 |
| [8] | ASTM D4294 -08a, Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry |
| [9] | ASTM D1945-07 Standard Test Method for Analysis of Natural Gas by Gas Chromatography,1-17 |
| [10] | ASTM D1298-05, Standard Test Method for Density, Relative Density (specific Gravity) or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer method, 1-6 |
| [11] | Abbas. Ammar S and Shubar Swasan D. A., Pyrolysis of high density polyethylene for the production of fuel like Liquid hydrocarbon, IJCPE,VOL 9, NO.1, march 2008, 27,28 |
| [12] | Sarker Moinuddin, Rashid Mohammad Mamunor,Rahman Md. Sadikur, Agricultural waste plastic convertion into high energy liquid hydrocarbon fuel by thermal degradation process, JPTAF, august 2011, 141-145, |
| [13] | Ademiluyi T, Adebayo. T A., Fuel gases from pyrolysis of waste polyethylene sachets,JASEM, vol 11(2), June 2007, 21-26 |
| [14] | UOP 539-97 ,Refinery Gas Analysis by Gas Chromatography, 1-17. |
| [15] | B.S. Bhal and Arun Bhal, Synthetic Polymer, Advance Organic Chemistry, 1293-1296. |
| [16] | CIPET, Plastic Industry –Statistics, Global Scenario, http://cipet.gov.in/plastics_statics.html |

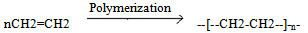 There are two types of plastic, Thermoplastic and Thermosetting plastic. Thermo plastic are the plastic that do not undergo chemical change in there composition when heated, some examples are polyethylene, polypropylene, polystyrene etc. Thermosetting plastics melts and take shape ones. Chemical reaction in thermosetting plastics are irreversible.Plastic to oil technology is a two fold transformation of non-recyclable plastic into useful commodities and creating a reliable source for an alternative energy from an abundant at no extra cost for feedstock. The conversion from polyethylene to oil is a depolymerisation process to convert scrap polyethylene into petroleum products. This process is utilized to convert long chain polymers to monomers that can be used to rebuild resins having the property and performance of virgin resins.In this paper the author have described the process of converting scrap polyethylene preferably low density polyethylene(LDPE) into useful gaseous and liquid hydrocarbon products (gasoline and middle distillate) through hydropyrolysis route. Pyrolysis is the thermochemical decomposition of organic material at elevated temperature in the absence of oxygen[2] .In hydropyrolysis process the pyrolysis process is carried out in the presence of water. The product obtained plastroleum (plastic+petroleum (derived from plastic)) through hydropyrolysis process have some distinct properties. The conversion of polyethylene generally low density polyethylene(LDPE) into useful hydrocarbon products will change the enviournmental concerns of soil and water pollutions due to landfills and sewage choking problems. This process also help in reducing the greenhouse gases in the enviournment such as NOx, SOx, CO2, CO etc. The aim of this research is to study the characteristic property of the products obtained by the low to medium high temperature hydropyrolysis of plastroleum products obtained from polyethylene. The enviournmental and economic feasibility of the products were also discussed in this paper. The depolymerisation and repolymerisation reaction carried out in the process were also studied. The chromatographic study of gases and the liquid hydrocarbon (naphtha) and the FTIR (Fourier Transform Infra Red Spectroscopy) of the plastroleum crude, naphtha and the middle distillate samples were also studied. The water in which the plastroleum is recovered is also analysed and the total oil content in it, is determined.
There are two types of plastic, Thermoplastic and Thermosetting plastic. Thermo plastic are the plastic that do not undergo chemical change in there composition when heated, some examples are polyethylene, polypropylene, polystyrene etc. Thermosetting plastics melts and take shape ones. Chemical reaction in thermosetting plastics are irreversible.Plastic to oil technology is a two fold transformation of non-recyclable plastic into useful commodities and creating a reliable source for an alternative energy from an abundant at no extra cost for feedstock. The conversion from polyethylene to oil is a depolymerisation process to convert scrap polyethylene into petroleum products. This process is utilized to convert long chain polymers to monomers that can be used to rebuild resins having the property and performance of virgin resins.In this paper the author have described the process of converting scrap polyethylene preferably low density polyethylene(LDPE) into useful gaseous and liquid hydrocarbon products (gasoline and middle distillate) through hydropyrolysis route. Pyrolysis is the thermochemical decomposition of organic material at elevated temperature in the absence of oxygen[2] .In hydropyrolysis process the pyrolysis process is carried out in the presence of water. The product obtained plastroleum (plastic+petroleum (derived from plastic)) through hydropyrolysis process have some distinct properties. The conversion of polyethylene generally low density polyethylene(LDPE) into useful hydrocarbon products will change the enviournmental concerns of soil and water pollutions due to landfills and sewage choking problems. This process also help in reducing the greenhouse gases in the enviournment such as NOx, SOx, CO2, CO etc. The aim of this research is to study the characteristic property of the products obtained by the low to medium high temperature hydropyrolysis of plastroleum products obtained from polyethylene. The enviournmental and economic feasibility of the products were also discussed in this paper. The depolymerisation and repolymerisation reaction carried out in the process were also studied. The chromatographic study of gases and the liquid hydrocarbon (naphtha) and the FTIR (Fourier Transform Infra Red Spectroscopy) of the plastroleum crude, naphtha and the middle distillate samples were also studied. The water in which the plastroleum is recovered is also analysed and the total oil content in it, is determined.









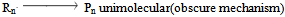
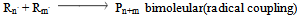
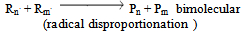
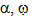 olefin,
olefin,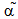 olefin and n-paraffin constituents. Polyaromatic components are produced on heating the polyethylene material at higher temperature[4] . At lower temperature the generation of lighter gaseous hydrocarbon product increases range from C1 to C5. The low temperature hydropyrolysis of polyethylene material enhances the production of plastroleum crude and hydrocarbon gases with. Prolonged heating of polyethylene material for longer time at specified controlled temperature helps the longer hydrocarbon chain to crack. Depolymerisation and repolymerisation of the cracked product into shorter chain and branched chain hydrocarbon products also increases the olefinic content. The increase in the olefin content and iso-paraffinic components increases the antiknocking properties of the product. The aromatic content increases with time and temperature which is due to the reaction of ethane and propene. It was observed that with higher temperature and longer pyrolysis time the heavier products decomposes to lighter one, because at higher pyrolysis time the carbon chain was exposed to more forceful thermal decomposition effect[11] . The residue material which is left in the bottom of the vessel contains, generally a black char material having components which are used as binders or additives and contaminants which enters in the waste polyethylene material as part of feedstock. These are generally residue containing calcium carbonates, sludge, heavy materials, metals, clay and carbon black. The above flow diagram shown in Figure 1 gives idea about the process of extracting plastroleum crude, gases, naphtha, middle distillate and heavy cut oil( in solid condensed form) (from left to right).
olefin and n-paraffin constituents. Polyaromatic components are produced on heating the polyethylene material at higher temperature[4] . At lower temperature the generation of lighter gaseous hydrocarbon product increases range from C1 to C5. The low temperature hydropyrolysis of polyethylene material enhances the production of plastroleum crude and hydrocarbon gases with. Prolonged heating of polyethylene material for longer time at specified controlled temperature helps the longer hydrocarbon chain to crack. Depolymerisation and repolymerisation of the cracked product into shorter chain and branched chain hydrocarbon products also increases the olefinic content. The increase in the olefin content and iso-paraffinic components increases the antiknocking properties of the product. The aromatic content increases with time and temperature which is due to the reaction of ethane and propene. It was observed that with higher temperature and longer pyrolysis time the heavier products decomposes to lighter one, because at higher pyrolysis time the carbon chain was exposed to more forceful thermal decomposition effect[11] . The residue material which is left in the bottom of the vessel contains, generally a black char material having components which are used as binders or additives and contaminants which enters in the waste polyethylene material as part of feedstock. These are generally residue containing calcium carbonates, sludge, heavy materials, metals, clay and carbon black. The above flow diagram shown in Figure 1 gives idea about the process of extracting plastroleum crude, gases, naphtha, middle distillate and heavy cut oil( in solid condensed form) (from left to right).











 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




