-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2013; 3(2): 45-54
doi:10.5923/j.ijee.20130302.02
Modeling Lateral Distribution of Heavy Metal and Bio-accumulation in Earthworm in the Varying Acidic Surface Horizon of Waste- Polluted Soil
Atulegwu Patrick uzoije1, Luke uzoigwe2, Erick otuonye3, Clifford O Kamalu4, Austine Onunkwo-Akunne5
1Department of environmental engineering, Federal University of Technology Owerri
2Department of Agricultural Engineering Imo state University Owerri
3Department of Statistics, Abia State University Uturu
4Department of Chemical Engineering, Federal University of Technology Owerri
5Department of Geology, Federal University of Technology Owerri
Correspondence to: Atulegwu Patrick uzoije, Department of environmental engineering, Federal University of Technology Owerri.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
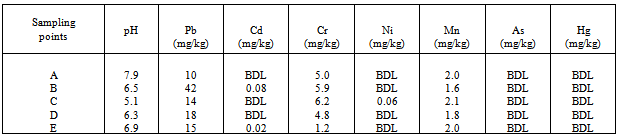
Heavy metal concentrations and its distributions in the soil have been a source of concern to soil usage, particularly to agriculture as concentration and distribution of heavy metal determine to a large extent, the soil quality and consequently that of the crops. The ability to quantify the amount of heavy metal in the soil is of immense importance to soil management. The use of accurate model is essential to estimate the actual soil heavy metal values and its distribution for efficient management. In this study, soil and earthworm samples of the battery-waste-polluted site and that of the background site were collected from five different locations(A,B,C,D and E) along the gradient of decreasing pollution .With five replicates each form one sampling location, twenty–five soil and fifty earthworms samples (two earthworms from each replicate) were collected using stratified random sampling technique. Lead(pb), Cadmium(Cd), Chromium(Cr), Nickel(Ni), Manganese(Mn), Arsenic(As), pH and Mercury(Hg) were analyzed using standard methods. The same process was replicated for the background site. Values of the heavy metals in soil and earthworms were natural and typical of Ameki-Nanka-soil formation. The average range of heavy metals concentrations in soil and earthworm samples from the polluted site were; pb(1025-695 mg/kg), Cd(11.34-6.3 mg/kg), Mn(290-81 mg/kg), pH(2.3-6.9mg/kg), Cr(185-3.7 mg/kg), Ni(12.87-1.7 mg/kg), As(72-4.5 mg/kg), Hg(1.7-0.002mg/kg) and pb(193-37.98 mg/kg), Cd(14.04-0.01 mg/kg), Mn(17.34-1.10mg/kg), pH(6.9-2.3mg/kg), Cr(8.45-0.01 mg/kg), Ni(1.41-0.03 mg/kg), As(0.75-0.01 mg/kg) , Hg(0.4-0.009mg/kg) respectively. Concentrations of heavy metals for soil and earthworm samples decreased along the gradient of decreasing pollution of the polluted site. Three models(Linear , Logarithmic and quadratic models) were developed to test their suitability to the data in which Ph was correlated with heavy metals. Inverse correlation was observed with coefficient R2 of between 0.77-0.95 and lowest percentage deviation of the field from the predicted values.
Keywords: Heavy Metals, Earthworm, Soil, Models, Pollution, Bio-Accumulation
Cite this paper: Atulegwu Patrick uzoije, Luke uzoigwe, Erick otuonye, Clifford O Kamalu, Austine Onunkwo-Akunne, Modeling Lateral Distribution of Heavy Metal and Bio-accumulation in Earthworm in the Varying Acidic Surface Horizon of Waste- Polluted Soil, International Journal of Energy Engineering, Vol. 3 No. 2, 2013, pp. 45-54. doi: 10.5923/j.ijee.20130302.02.
Article Outline
1. Introduction
- Heavy metals are essential for optimum crop production . They can also act as toxicants to soil and crops at elevated concentrations. Heavy metals such as Mn, Ni, Cd etc are required at micro level for optimum crop performance[1] and[2]. In most cases they play key roles in development and growth of crop plants[3]. At high concentration, the same metals can result to stunted growth of crops and consequently adverse affect on the final consumer of these crops[4]. Aside the land discharge of chemical wastes which are usually associated with heavy metals, the major pathways of heavy metal into the soil are mainly through application of compost and chemical fertilizer[5]. There was also an evidence that heavy metals could enrich the agricultural soil and pose environmental and health risks of the plants through plant up-take of the discharged waste water and sludge[6] and[7] . In the tidal and fresh waters, heavy metals rapidly move from the water body to deposit onto the sediments[8] and[9]. Variations in thecharacteristics of these heavy metals were evident in their varying mobility rates and distributions. In a study of evaluation of heavy metals in core sediments of southeast cost of India, there were heavy presences of lead, cadmium and zinc at the bottom layer of the sediments whereas chromium and copper dominated the upper layer. There was also a measure of variability in the heavy metal distributions within the soil matrix. Mustapha et al 2010[10] observed that values of extractable Zn, Cu, Fe and Mn were predominant in the respective depths of 0.48 - 0.75, 0.18 - 0.26, 18.40 - 21.91 and 30. 54 - 38.58 of a natural sandy loam soil of northern Nigeria. In a cassava-mill–effluent polluted eutric - tropofluvent soil Cn and Pb heavily polluted 70-100cm depth of the soil matrix[11]. Lateral variability of Zn at 5meters interval was observed in an oil polluted soil[12]. According to[13], the apparent distribution variation observed was as a result of different soil physical, chemical and biological properties. In their study,[14] observed that high accumulation of heavy metals affected adversely the biological and physio-chemical status of the soil. These manifested in low soil carbon mineralization rate, impairment of respiration of soil microbes and low soil microbial biomass carbon. In the physio-chemical sense, high heavy metal concentration upsets the pH, salinity and electrical conductivity (EC) balance of the soil[15]. Aside the physiochemical properties of soil, bio-accumulation of heavy metals on certain invertebrates could be a reliable indicator of heavy metal toxicity of the environment[16],[17] and[18]. Owing to the ecological importance of earthworms in most temperate an tropical soils, earthworms are used as bio-indicators to assess the bioavailability of heavy metals in terrestrial environment[19],[20]. In an attempt to purify itself, agricultural soils infested with heavy metal-based waste as in the case of Ameki/Nanka soil formation of Nnewi town, have the tendency to attenuate the high concentration of heavy metal by naturally producing alkaline substances to counter the effects of low pH occasioned by high concentration of heavy metal[21] Nnewi town houses one of the major small and medium industrial clusters in southeastern Nigeria. These industrial wastes, mainly associated with heavy metals, are discharged into the environment without adequate treatment. The car battery manufacturing industry is one of such industries. The industry practices open dumping of spent acid solution and the discarded battery casing in a used borrow pit that stretches up to one and half a kilometer. The aggressive impact of the acid-based waste which resulted to clay lessivage[22] led to sandiness of the site soil. The prevalent state of the soil may lead to reduction in soil bulk density, increase in soil porosity , possible infiltration of pollutants and aquifer pollution[23] and consequent land slid observed in the site. In order to study the distribution of these heavy metals, mathematical models were used. Most of these models were predictive[24] to forecast the level of heavy metal pollution in an environment after certain distance and period . This is relevant for the purposes of determining the lateral distributions of heavy metals in a typical Ameki / nanka soil , to ascertain its level of heavy metal pollution distances away from the study site and for making sound environmental decisions. There is little information on the impact of car battery industry on the environment, particularly on the extent to which high load of heavy metal has been laterally distributed on Ameki/Nanka soil formation of Nnewi town . The study will therefore examine the spatial distribution of heavy metal on the lateral dimension of the dump site using both physical and boimonitoring approach. Also, the study will develop statistical models capable of approximating the concentration of heavy metals in the dump site.
2. Materials and Methods
2.1. Description of the Study Area
- The study area lies within latitude 50 58’N and longitude 060 55’ at an average elevation of 90.83m (GPS reading) and is underlain by cretaceous to recent sedimentary formations of Anambra basin of Nigeria which have varying aquifer potentials. The basin is dominantly filled with clastic sediments ranging from upper campanian to recent in age . Geology of Nnewi area is characterized by Ameki Formation. See figures i and ii for map of the study area and its geological map respectively.Greater part of the study area is made of Plateau and ridges . This is a part of the higher Plateaus that extends from Enugu towards South east of Anambra State of Nigeria, and has the characteristics of dissection and marginal ridges that consists of small low lands.The soil is consist of moderately drained sands, silt and sandy clay with top layer of black to brownish humus and laterite.
2.2. Soil Sample Collection
- The entire study area was divided into two broad morphological units, the background or control and the dumpsite. The dumpsite was further divided into five units and the units were designated as units A,B,C,D and E downstream. the five sampling units of A, B, C, D and E stretched up to 1500m which represents the size of the study . Sampling units A B and C constitute the portions of the study site that receive the highest quantity of the waste while the quantity of waste reduces at the portion of units D and E . Units A, B and C were measured to 250m each in size. The sizes of units D and E which receives less waste load were measured to be 300 and 400m respectively. A transect was drawn to link the five units. Within each unit, soil samples were collected from 20cm deep with the help of soil auger . Adopting the stratified random sampling technique, the sample collections were done in fives replicates in each unit, making a total of 25 soil samples. SOLAAR UNICAM 969 atomic absorption spectrophotometer (AAS) was adopted to analyze the metals (lead, cadmium, chromium, nickel, manganese, arsenic and mercury) as reported by[22]. Soil samples also taken from the background unit 500m away from the dumpsite, adopting the same sampling technique were equally analyzed.
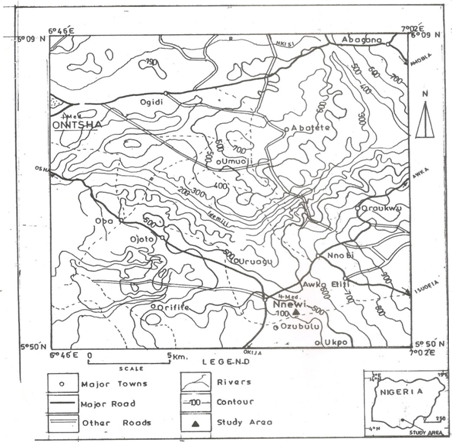 | Figures i. Topographical map of study area |
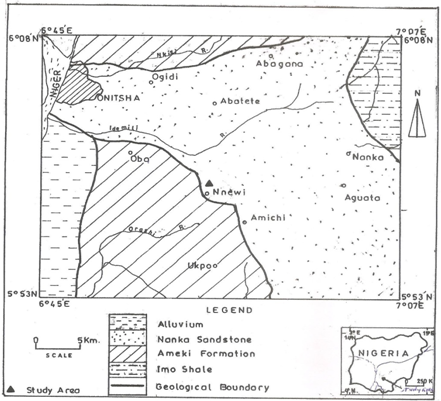 | Figure ii. Geological mop of the study area |
2.3. Earthworm Collection and Heavy Metal Determination
- Samples of earthworm were collected from all the units where the soil samples were collected. This implies that a total of 25 worm samples were collected each from both the background and polluted site. The washed worm samples were digested in 2.5ml concentrated nitric acid and heated to dryness with a hot plate. The dried earth worms were crushed, re-dissolved in 1.5 nitric acid and the solution was filtered off into a volumetric flask. After which the filtrate was made up to 50ml with distilled water. From there, samples were taken for heavy metal analysis using AAS
2.4. Model Approach
- In science and engineering, many forms of models have been used to find the relationship between the soil and the environment. For instance Philip model, provides infinite series solution to solve the non-linear partial differential Richard equation describes transient fluid flow in a pororus medium[23] and[24]. Horton model provides a three semi - parameter infiltration model to estimate the runoff infiltration into the subsoil[25]. Regression models have been widely used in prediction of soil attributes especially the pollution level, because of their easiness and wide availability. Regression models provide global model process and create a single regression equation to represent the process[26]. In this study regression models involving Linear, quadratic, polynomial, logathimic and power functions will be adopted to predict the values of heavy metals in relation to other(s). In other words, the models explain the variation in one variable base on the variations on one or more other variables[27]. Dependent variable is that variable which the variation explain while the variables which are used to explain the variation is referred to as the independent variable .
2.5. Model Validation
- The models will be validated by direct analysis and comparison of the values of experimented and predicted. The analysis and comparison of the two values reveal the percentage deviation(%DV) expressed as
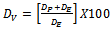 | (1) |
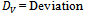
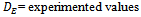
3. Results and Discussions
3.1. pH and Heavy Metal Distributions on the Background Units
- Table 1 shows the values of the heavy metal concentrations in the background (control)morphological unit which is 500m from the dump site. In this unit, Ni, As, and Hg were below detectable limit , whereas the values lower than the recommended USEPA standard were observed for Cd ,Pb, Cr, and Mn. The low heavy metal concentration values observed at the background study unit was apparently due to far proximity of the unit to the dumpsite. Again there was also a likelihood of natural depletion of these metals at the top soil through alluviation process. The study of alluvaition- illuviation process of heavy metals through eutric - tropofluvent soils carried out by[28] gave credence to this observation . Significant depletion of Fe and Al at the topsoil of an oil polluted Arenic paledudult reported by[29] also corroborated the present study. The pH levels of all the samples from different sampling points at the background unit, lie in the range of basic to neutral to slightly acidic. On the average, the pH value at the background unit was observed to be moderately acidic with an average pH value of 6.8 which was typical of Ameki/Nanka formation as observed by[30] and[31].
3.2. pH Levels and the Heavy Metal Distributions on the Sampled Polluted Sites
- Figure iii presents the average distribution values of pH and heavy metal in the polluted site at various sampling points . The figure generally revealed that the pH of the soil samples increased downstream, suggesting a reduced impact of the battery manufacturing waste away from unit A of the dumpsite. This implies that pH of location A is lower than that of B and pH increased in that order to its peak at location E. The observed increase in pH value away from the waste discharge point accounts for the reduction of waste concentration due to increase in proximity of the locations from the discharge units and the natural attenuation of the waste with time[32] . The study also shows that soil samples were observed to be generally acidic with mean acidic pH value of 3.9 . The low pH level observed at locations A , B and C is attributed to acid based solid and liquid waste discharged from the factory . Another factor could be as a result of microbial conversion of sulphur to sulphonic acid at the upper layer of the soil through anaerobic process and decomposition of organic residues to organic acid which were engendered by acidic environment[33]. The low pH range observed could possibly be due to heavy presence of micro-organisms which thrive within acidic environment. These micro-organisms, especially thiobaccillus and thaoxidants performs(conversion of sulphur to sulphonic acid) optimally at pH range of between 2-4[34]. With the presence of these organisms, the soil environment is bound to remain acidic[35]. Observations of these researchers also corroborated the present study. In this regard, these micro-organisms mentioned , perhaps capitalized on the low acidic environment of the study area to further reduce its acidity by catalyzing the conversion process of sulphur to sulphonic acid.
 | Figure iii. Distribution of heavt metals with respect to sample locations |
|
3.4. Heavy Metal Distribution In Various Sampling Polluted Sites
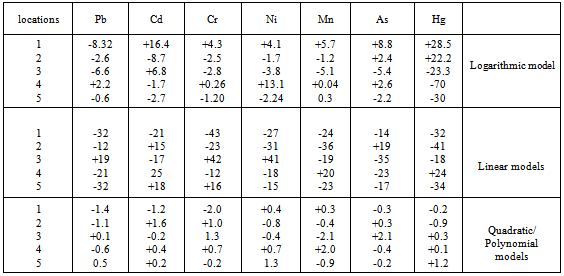
- Also, fig iii glaringly showed that heavy metal distribution responded sufficiently to spatial variability of pH. Observations from the results revealed an inverse trend of relationship between pH and heavy metal variation .The concentration of the sampled heavy metals decreased as the distance from the waste dump site increased downstream, showing glaring spatial variability of the measured parameters. This trend of concentration gradient explains the classical natural attenuation tendency occasioned by even mass flow of pollutant out of a point source[36]. Amadi et al 1993[37] had attributed such natural attenuation to alluviation and alivation process . In all the sampling units lead(Pb) has the highest concentration values followed by manganese (Mn), chromium (Cr)and Asenic(As) with cadmium (Cd), Nickel(Ni) and mercury (Hg) having the least value. This result implies that the soil was substantially contaminated with lead. There were apparent differences in heavy metal values at various study units. Highest values of heavy metals were recorded at unit A, followed by unit B and the values decreased to the lowest level at unit E. The decrease in the values of the heavy metal with respect to units is represented thus; A>B>C>D>E. The dominance of lead in the present study confirms that it is the major component of the battery cell. Also, the already low pH value of the soil medium due to discarded acid battery solution aggravated the heavy presence of lead .This tends to favour low migration of lead[50]. The trend of heavy metal distribution in the sampling points is generally shown as follows; pb>Mn>Cr>As>Cd>Hg. However, distribution of pb and Mn in all the sampling points were found to be above maximum tolerable concentration level provided by USEPA 1993, whereas Cr, As, Cd and Hg concentration values were below the limit hence constituting minimal environmental threat. Again, the high lead(Pb) and manganese (Mn) observed in this study is in response to low pH value that characterized the topsoil[38],[39] and[40]. Earlier,[41],[42] and[43] observed that pb and Mn experienced low stability, mobility and inability to form complexes at low acidic level while there was significant accumulation of Cd to the soil at low acidic range. This observation is at variance with cadmium distribution behavior of the present study. There was no significant accumulation of cadmium in the sampling sites rather; the sites witnessed low cadmium values. The variance might stem from differences in soil and plant morphologies of the study areas from those of the previous studies. For instance, metal distribution within a soil matrix is highly a function of soil morphology and ability of the vegetal cover on the land surface to uptake the heavy metal [44]. Similarly, Dinikya, and Areola 2010[45] reported that Fe and Mn saturation levels vary widely between and among crop types but in general, the highest levels of heavy metal accumulation were found under olive, tomato and spinach. Also, the high Pb and Mn values observed at the dumpsites could explain the low plant uptake of these metals and perhaps the seemingly lower Cd values of the dumpsites relative to the background could be as a result of high Cd plant uptakes[46]. These observations explained the apparent high Pb and Mn concentration and low Cd concentration of the present study.
3.5. Values of Heavy Metal Accumulation in Earthworms
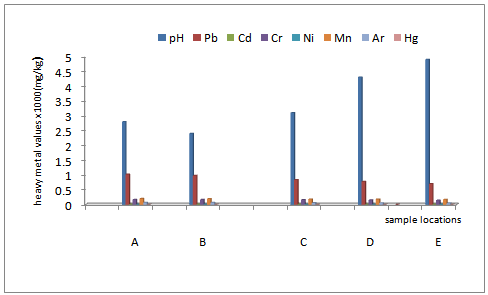
- Heavy metal accumulation in the worms collected from various sampling points are shown in figure iv. The amount decreased along the distance, that is, from locations 1 through 5. The concentrations of heavy metals extracted from the earthworms collected in location 1 of the polluted site were found to have the highest value. The concentrations decreased downstream with the least value observed in location 5. The locations with respect to the extracted heavy metals distribution were ranked in this order; 1›2›3›4›5 and was explicitly show- cased in figure iv. This trend of distribution was attributed to the proximity to the waste -received -location. The earthworms in location 1 accumulated the highest amount of all the heavy metals, corresponding to the metal speciation pattern in the contaminated soil earlier observed. This is apparently due to high concentration levels of heavy metal bio-accumulation recorded in the soil samples of the location. Heavy metal bioaccumulation in the earthworms reduced downstream, that is, from location 1 down to 5. Also, within the contaminated sites, extreme low pH and high metal concentration could be responsible for low abundance of earthworms observed in location 1 compared to other locations. The ones found in location 1 were blackish and small in size. At location 5 which was at the extreme location of the waste- received -location ( location 1) and where the pH was relatively high and heavy metal concentrations relatively low, high abundance of big sized earthworms was observed. This implies that earthworms are sensitive to the soil physiochemical imbalance and also an index for evaluating toxicity of soil as earlier observed by[47on the lethal effects of low pH on the soil invertebrates and fauna. The study also assessed the percentage bioaccumulation of various heavy metals in the earthworms found at different sampling locations. In location 1, bioaccumulation of Pb recorded the highest value, followed by Cd with Hg having the least value. Percentage Bioaccumulation of heavy metals by earthworms in locations 1-5 was ranked as; Pb >Cd>Ni> Cr>As>Mn>Hg, Pb>Cd>Ni>Cr>As>Mn>Hg, Pb>Cd>Cr> As>Ni>Hg>Mn, Cr>As>Pb>Ni>Mn>Hg>Cd, Cr>As>Ni> Pb>Mn>Hg respectively. The apparent high accumulation of Cd in virtually all the locations could be attributed to induction of cadmium-binding protein with thecharacteristics of metallothioeins as corroborated in the work of[48]. This could also be attributed to low metabolic and excretion of Pb and Cd by the earthworms. Spurgeon and Hopkin (1999)[49]; Weltje (1998)[50], and Buatier et al., 2001 )[51] had observed that the earthworm gut could modify the mobility of metals due to change in pH, making most xenobiotic metals such as Pb and Cd very insoluble in the tissues of the earthworm and difficult to excrete. Also, Cd and Pb are more mobile in soil than other metals, resulting to high bioavaliabilty to earthworms. On the contrary, earthworms have high metabolic and defecation rate for some metals, leading to their easy excretion[52]. This observation could explain the apparent low percentage bioaccumulation of As and Hg recorded in most sampling locations of the study area. Low bio availability of these metals to earthworms could equally attribute to their low tissue bioaccumulation.
 | Figure iv. percentage bio-accumulation of heavymetals in the earthworm |
3.6. Heavy Metal Distribution Prediction Models(NMDPM)
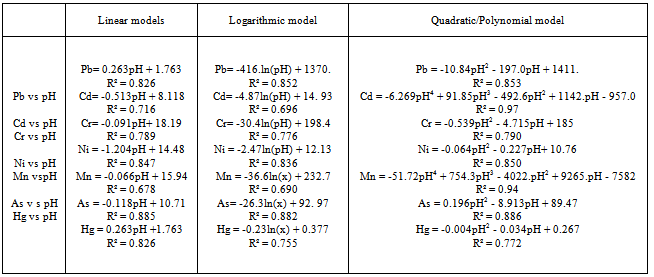
- Statistical models were developed to predict the levels of heavy metal distribution in relation to Ph values using Microsoft Excel. The data were tested with Linear, logarithmic,quadratic and polynomial models to determine their suitability to the data. The models were based on regression analysis in which the explanation of one variable is based on the variations of one or more of the variables. The variables which explains the variation in a relationship is called the dependent variable while the independent variable predicts the dependent variable. In this study, pH was chosen as an independent variable due to its major factor in determining the concentration and distribution of heavy metals. Therefore, pH predicted the concentrations of pb, Cd, Cr, Ni, Mn, As and Hg in the soil. The Linear, logarithmic, quadratic and polynomial regression models developed, fitted the relationships with regression co-efficients (R2) ranging between 0.68-0.94 to corroborate the dependency of heavy metal concentration and distribution on pH as observed by some researchers[53] and[12]. Quadratic / polynomial models gave the highest prediction of heavy metal concentration and distribution with R2 ranging from 0.79-0.94. Therefore the most statistically suited parametric model for this study was selected to be Quadratic/polynomial based on its high significant regression co-efficient(R2) observed . This was followed by the logarithmic model with R2 of between 0.69-0.85 and the least was the linear model with R2 of between 0.68-0.85. The highest prediction of heavy metal concentration and distribution by quadratic / polynomial was consistent with the findings of Rajarathinam and parmar[25] . The models are shown on table 2.
|
|
4. Conclusions
- Modeling of Heavy Metal Distribution in the Varying Acidic Surface horizon of Battery –Waste- Polluted -Ameki-Nanka -Soil -Formation and bio-accumulation of heavy metals in the earthworm have been studied. The study showed that heavy metal distribution in the background unit was natural and typical of Ameki-Nanka soil-formation. That of the polluted site showed apparent heavy metal pollution with the metals concentration distribution decreasing along the gradient of decreasing pollution of the site. The earthworms bio-accumulated more of the lead and cadmium. The model developed to predict the values of heavy metals in relation to varying acidity level of the soil showed that quadratic/polynomial models better fits the data than the other models. After validating the model, the experimental and predicted showed little discrepancies with quadratic/polynomial model.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML