| [1] | Lim,L.H.,Macdonald,D.G and Hill,G.A.,Immobilized Barley α-Amylase for Hydrolysis of Starch Particles.Final report for Saskatchenan Agriculture Development and Food under contract 20000155,(2001) |
| [2] | Stern, N;The Economics Of Climate Change. The Stern Review. CabinetOffice-HMTreasury.Cambridgeuniversitypresshttp://Www.Hm-Treasury.Gov.Uk/Independent_Reviews/Stern_Review_Economics_Climate_Change/Stern_Review_Report.Cfm (2006) |
| [3] | Houghton,J.T, DingY, Griggs,D.J, Noguer M,Van Der Linden,P.J., Xiaosu D(Eds)(2001)Climate Change :The Scientific Basis.Contributionof Working Group I To The Third Assessment Report of The Intergovernmental Panel on Climate Change(IPCC).Canbridge UniversityPress,UK,944,(2001). |
| [4] | Kumarn.,Sharma,P.B., “Jatropha Curcus - A Sustainable Source for Production Of Biodiesel”, Journal Of Scientific And Industrial Research, Vol. 64(11), Pp. 883-889,(2005). |
| [5] | Shah,S Gupta,M.N, “Lipase Catalyzed Preparation of Biodiesel From Jatropha Oil in a Solvent Free System”, Process Biochemistry, Vol. 42(3), Pp.409-414,(2007). |
| [6] | Mittelbach M, Pokits B And Silberholz A Production and Fuel Properties of Fatty Acid Methyl Esters from Used Frying Oil. Liquid Fuels From Renewable Sources: Proc. of An Alternative Energy Conf., St. Joseph. Mich. ASAE. Pp: 74–78,(1992). |
| [7] | Alcantara R, Amores J, Canoira L, Hidalgo E, Franco MJ, Navarro A Catalytic Production of Biodiesel From Soybean Oil, Used Frying Oil and Tallow. Biomass Bioenerg. 18, 515 – 527,(2000). |
| [8] | Canakci M and Gerpen J.V Biodiesel Production from Oils and Fats with High Free Fatty Acids. Trans. ASAE. 44, 1429-1436,(2001). |
| [9] | Dorado M.P, Ballasteros E, Almeida J.A, Schellert C, Lohrlein H.P And Krause R (2002) An Alkali Catalyzed Transesterification Process For High Free Fatty Acid Waste Oils. Trans. ASAE. 45,525- 529,(2002). |
| [10] | Olsen, C.S. A Qualitative Assessment of The Sustainability of Commercial Non-Timber Forest Product Collection in Nepal. Forestry Discussion Paper 12, Royal Veterinary and Agricultural University, Copenhagen, Pp. 30,(1997). |
| [11] | Arjun B. C, Martin S. T, Suzanne M. B, Chris W.K and Rafiqul I.M , Non-Edible Plant Oils as New Sources for Biodiesel Production, Int. J. Mol. Sci. 9, 169-180,(2008). |
| [12] | Prokop, T., Personal Communication, Imperial Western Products,14970 Chandler St., Coachella, CA 91720,(2002). |
| [13] | Lott, M., (2002). Personal Communication, QSS Group Inc., 4500 Forbes Boulevard, Suite 200, Lanham, MD 20706,(2002). |
| [14] | Demirbas, M., Balat, M., Recent Advances on the Production and Utilization Trends of Bio-Fuels: A Global Perspective. Energy Conversion and Management 47, 2371–2381,(2006). |
| [15] | Marchetti, J.,and Errazu, A., Techno-economic Study of Supercritical Biodiesel Production Plant. Energy Conversion and Management 49, 2160–2164 (2008). |
| [16] | Mohibbe Azam, M., Waris, A., Nahar, N., Prospects and Potential of Fatty Acid Methyl Esters of Some Non-Traditional Seed Oils for use as Biodiesel in India.Biomass and Bioenergy 29, 293–302,(2005). |
| [17] | Sarin, R., Sharma, M., Sinharay, S., Malhotra, R., Jatropha–Palm Biodiesel Blends: An Optimum Mix For Asia. Fuel 86, 1365–1371,(2007). |
| [18] | Zhang, Y., Dubé, M., Mclean, D., Kates, M., Biodiesel Production from Waste Cooking Oil: 2. Economic Assessment And Sensitivity Analysis. Bioresource Technology 90, 229–240,(2003) . |
| [19] | www.Biofuels.Coop |
| [20] | Divya,B;andTyagi,V.K,(2006),Biodiesel:Source,Production,Composition,Properties and Its Benefits.J.Oleo Sci.,Vol.55,No.10,487-502,(2006). |
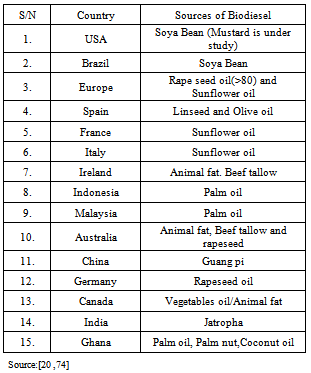
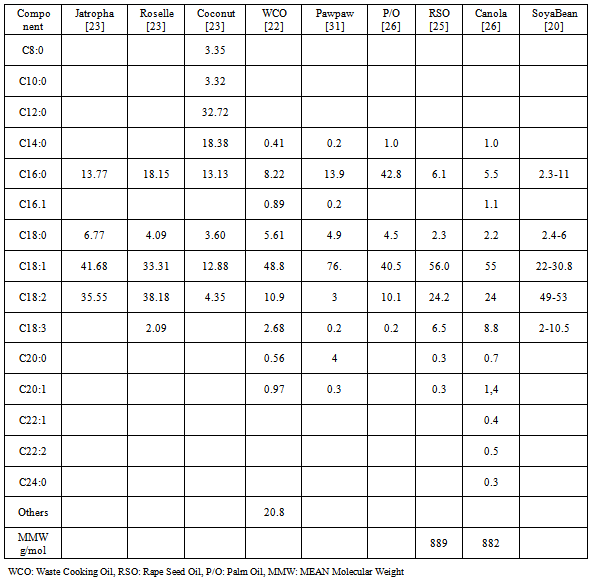
| [21] | Owolabi R U, Osiyemi N A, Amosa M K And Ojewumi M E, Biodiesel from Household/Restaurant Waste Cooking Oil (WCO), J Chem Eng Process Technology, 2:4,(2011). |
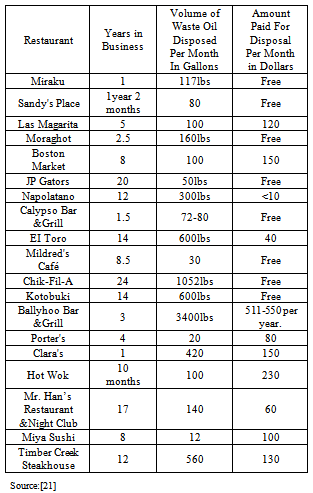
| [22] | Issariyakul, T.; Kulkarni, M.G.; Dalai, A.K.; Bakhshi, N.N. Production of Biodiesel Form Waste Fryer Grease Using Mixed Methanol/Ethanol System. Fuel Processing Technology , 88, 429- 436,(2007). |
| [23] | Piyanuch, N And Sasiwimol, W; Biodiesel Production From Mixtures of Vegetable Oil and Used Cooking Oil, International Conference on the Role of Universities in Hands-On Education ,Rajamangala University of Technology Lanna, Chiang-Mai, Thailand,(2009). |
| [24] | Mohd Ghadafi,I, Biodiesel Production from Waste Cooking Oil Via Single Steps Tranesterification Process with the aid of Sodium Methoxide as a Catalyst,B.Sc Project,Faculty Of Chemical & Natural Resources Engineering Universiti Malaysia Pahang (2008). |
| [25] | Rice,B ,Frohlich,A,Leonard,R (1997), Bio-Diesel Production Based on Waste Cooking Oil: Promotion of The Establishment of an Industry in Ireland,Altener Contract No. Xvii/4.1030/Al/77/95/Irl,(1997). |
| [26] | Leung, D.Y.C.; Guo, Y. , Transesterification of Neat and Used Frying Oil: Optimization for Biodiesel Production. Fuel Processing Technology 87, 883-890,(2006). |
| [27] | Kumar M.S., Ramesh, A., Nagalingam, B., An Experimental Comparison of Methods to use Methanol and Jatropha Oil in a Compression Ignition Engine, Biomass and Bioenergy, 25, 309-318,(2003). |
| [28] | Dorado, M.P., Ballesteros, E., López, F.J. And Mittelbach, M., Optimization of Alkali-Catalyzed Transesterification of Brassica Carinata Oil for Biodiesel Production, Energy & Fuels, 18,7–83,(2004). |
| [29] | Arjun, B. C., Chris W.K And Rafiqul I.M ,Waste Cooking Oil as an Alternative Feedstock for Biodiesel Production, Energies , 1, 3-18,(2008). |
| [30] | Kinney A.J, Clemente T.E Modifying Soybean Oil For Enhanced Performance In Biodiesel Blends. Fuel Process Technol 86(10):1137–1147,(2005) |
| [31] | Ghazali,H.M; Puangsri,T; Abdulkarim,S.M;(2004). Properties of Carica Papaya.(Papaya) Seed Oil Following Extractions using Solvent and Aqueous Enzymatic Methods, Journal Of Food Lipids, 12, 62–76,(2004). |
| [32] | Gerpen, J.V. “Biodiesel Processing and Production”, Fuel Processing Technology 86 1097– 1107, (2005). |
| [33] | Kapilakarn K And .Peugtong. A., A Comparison of Costs of Biodiesel Production From Transesterication International Energy Journal 8 , 1-6,(2007). |
| [34] | Darnoko, D. And M. Cheryan., Kinetic of Palm Oil Transesterification in a Batch Reactor. JAOCS 77(12):1263-1267,(2000). |
| [35] | Fangrui Ma And Hanna, M. A. Biodiesel Production: A Review, Bioresource Technology 70:1-15,(1999). |
| [36] | Tongurai, C.; Klinpikul, S.; Bunyakan, C. And Kiatsimkul, P. Biodiesel Production From Palm Oil,Songklanakarin J. Sci. Technol, 23(Suppl.): Oil Palm:831-841,(2002). |
| [37] | Noureddini, H.and D. Zhu. Kinetic of Transesterification of Soybean Oil, JAOCS 74 (11): 1457-1463,(1997). |
| [38] | Berrios M, Siles J, Martin M.A and Martin A .A Kinetic Study of the Esterification of Free Fatty Acids (FFA) in Sunflower Oil. Fuel. 86, 2383-2388,(2007). |
| [39] | Kraai G.N, Winkelman J.G.M, De Vries J.G And Heeres H.J. Kinetic Studies on the Rhizomucor Miehei Lipase Catalyzed Esterification Reaction of Oleic Acid with 1-Butanol in Biphasic System. Biochem Engg. J. 41, 87–94,(2008). |
| [40] | Singh A.K and Fernando S.D. Reaction Kinetics of Soybean Oil Transesterification using Heterogenous Metal Oxide Catalysts. Chem. Eng. Technol. 30, 1716– 1720,(2007). |
| [41] | Komers,K.,Skopal,F.,Stloukal,R.,Machek, Kinetics and Mechanism of the KOH Catalyzed Methanolysis of Rapeseed Oil for Biodiesel Production,Journal of Lipid Science and Technology,Vol.104,728-737,(2000). |
| [42] | Kamini,N.R.,and Lefugi,H., Lipase Catalysed Methanolysis of Vegetable Oil in Aqueous Medium by Cryptococus Spp.S-2,Process Biotecnol.,Vol.37,405,(2001). |
| [43] | Meher, L.C., Sagar, D.V., Naik, S.N., Technical aspects of Biodiesel Production by Transesterification—A Review, Renewable and Sustainable Energy Reviews, 10(3),248-268 (2006a). |
| [44] | Meher, L.C., Sagar, D.V., Naik, S.N., Optimization of Alkali-Catalyzed Transesterification of Pongamia Pinnata Oil for Production of Biodiesel, Bioresource Technology, 97,1392-1397,(2006b). |
| [45] | Zheng, S.; Kates, M.; Dube, M.A.; Mclean, D.D. Acid-Catalyzed Production of Biodiesel from Waste Frying Oil. Biomass And Bioenergy,30, 267-272,(2006). |
| [46] | Jitputti J, Kitiyanan B, Rangsunvigit P, Bunyakiat K, Attanatho L, Jenvanitpanjakul P Transesterification of Crude Palm Kernel Oil and Crude Coconut by different Solid Catalysts. Chem Eng J 116:61–66,(2006). |
| [47] | Hossain,A.B.M.S., and Boyce,A.N., Biodiesel Production from Waste Sunflower Cooking Oil as an Environmental Recycling Process and Renewable Energy, Bulgarian Journal Of Agricultural Science, 15 (No 4), 312-317,Agricultural Academy,(2009). |
| [48] | Palligarnai T. V and Michael Briggs, Biodiesel Production—Current State of the Art and Challenges, J Ind Microbiol Biotechnol.(2008). |
| [49] | Chang, H.M, Liao, H.F, Lee, C.C, Shieh, C.J Optimized Synthesis of Lipase-Catalyzed Biodiesel by Novozym 435. J Chem Technol Biotechnol 80(3):307–312,(2005). |
| [50] | De Oliveira D, Di Luccio M, Faccio C, Dalla Rosa C, Bender J.P,Lipke N, Menoncin S, Amroginski C, De Oliveira J.V, Optimization of Enzymatic Production of Biodiesel from Castor Oil in Organic Solvent Medium. Appl Biochem Biotechnol 113– 116:771–780,(2004). |
| [51] | Fukuda H, Kondo A, Noda H , Biodiesel Fuel Production By Transesterification of Oils. J Biosci Bioeng 92(5):405–416,(2001). |
| [52] | Lai C.C, Zullaikah S, Vali S.R, Ju Y.H , Lipase-Catalyzed Production Of Biodiesel From Rice Bran Oil. J Chem Technol Biotechnol 80(3):331–337,(2005). |
| [53] | Noureddini H, Gao X, Philkana RS ,Immobilized Pseudomonas Cepacia Lipase For Biodiesel Fuel Production From Soybean Oil. Bioresour Technol 96(7):769–777,(2005). |
| [54] | Iso M, Chen B.X, Eguchi M, Kudo T, Shrestha S ,Production of Biodiesel Fuel from Triglycerides and Alcohol using Immobilized Lipase. J Mol Catal B Enzym 16(1):53–58,(2001). |
| [55] | Soumanou M.M and Bornscheuer U.T, Improvement in Lipase Catalyzed Synthesis of Fatty Acid Methyl Esters From Sunflower Oil. Enzyme Microb Technol33(1):97–103,(2003). |
| [56] | Deng L, Xu X.B, Haraldsson G.G, Tan T.W, Wang F Enzymatic Production Of Alkyl Esters Through Alcoholysis: A Critical Evaluation Of Lipases And Alcohols. J Am Chem Soc 82(5):341–347,(2005). |
| [57] | Matsumoto T, Takahashi S, Kaieda M, Ueda M, Tanaka A, Fukuda H, Kondo A Yeast Whole-Cell Biocatalysts Constructed by Intracellular Overproduction of Rhizopus Oryzae Lipase is Applicable to Biodiesel Fuel Production. Appl Microbiol Biotechnol 57(4):515–520,(2001). |
| [58] | Chen J.W, Wu W.T Regeneration of Immobilized Candida Antarctica Lipase for Transesterification. J Biosci Bioeng 92(2):231–237(2003). |
| [59] | Xu Y.Y, Du W, Zeng J, Liu D.H ,Conversion of Soybean Oil to Biodiesel Fuel using Lipozyme TL 1M in a Solvent-Free Medium. Biocatal Biotransformation 22(1):45–48(2004). |
| [60] | Lai C.C, Zullaikah S, Vali S.R, Ju Y.H . Lipase-Catalyzed Production of Biodiesel from Rice Bran Oil. J Chem Technol Biotechnol 80(3):331–33,7(2005). |
| [61] | Srivathsan,V.R, Srinivasan,L.N,Karuppan,M, An Overview Of Enzymatic Production Of Biodiesel, Bioresour. Technol. (2007), Doi:10.1016/J.Biortech.2007.04.060,(2007) |
| [62] | Ma, F., Hanna, M.A., Biodiesel Production: A Review. Bioresour. Technol. 70, 1–15,(1999). |
| [63] | Tiwari, A.K., Kumar, A., Raheman, H; Biodiesel Production from Jatropha Oil Jatropha Carcus) with High Free Fatty Acids: An Optimized Process. Biomass and Bioenergy, Pp569 – 575,(2008). |
| [64] | Alamu, O.J; Akintola,T.A; Enweremadu C.C And Adeleke .A.E(2008) Characterization of Palm-Kernel Oil Biodiesel Produced through NaoH-Catalysed Transesterification Process, Scientific Research and Essay Vol.3 (7), Pp. 308-311,(2008). |
| [65] | Reddy, J.N and Ramesh, A. Parametric Studies for Improving the Performance of a Jatropha Oil fuelled Compression Ignition Engine. Renewable Energy, 31, 1994-2016,(2005). |
| [66] | Sandip K .H and Ahindra Nag,.Utilization 0f Three Non-Edible Vegetable Oils for the Production of Biodiesel Catalysed by Enzyme, The Open Chemical Engineering Journal, 2, 79-83,(2008). |
| [67] | Biodiesel Report;Natonal Biodiesel Board,Jefferson City, MO.(1996). |
| [68] | Noureddini,H. US patent.174 501,(1997). |
| [69] | Tickell, J., From the fryer to the Fuel Tank:The Complete guide to using Vegetable Oil as an alternative Fuel. 3rd edition, Tickell Energy Consulting, Tallahassee, Florida. P. 36, 5254,(2000). |
| [70] | Anjan, B.K P., Rachna, C, Azeez P.A Biodiesel: Freedom from Dependence on Fossil Fuels. Nature Precedings : doi:10.1038/npre.2008.2658.1,(200) |
| [71] | Sudarsan KG and Anupa m a P M (2006). The relevance of biofuels. Current Science. 90 (6): 748749,(2006). |
| [72] | Belewu,,Challenges of Biofuel Production inNigeria.Seminar presented at Fountain university,Osogbo,Nigeria,(2011). |
| [73] | Olatunji,O.M , Akor, A.J , Abowei, J.F.N and Akintayo, C.O. Transesterification for the preparation of Biodiesel from Crude Oil of Milk Bush , Research Journal of Environmental and Earth Sciences 3(4): 358-363 , (2011) |
| [74] | Duku, M. H., Status of Biofuels Development in Ghana. ICS-UNIDO-MPOB, Workshop on Biofuels from Palm Oil: Emerging Technologies and their Assessments, Kuala Lumpur, Malaysia, (2007) |
| [75] | Ojolo,S.J , Ogunsina, B.S , Adelaja, A.O Ogbonnaya ,M , Study of an Effective Technique for the Production of Biodiesel from Jatropha Oil, Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS) 2 (1): 79-86 ,(2011) |
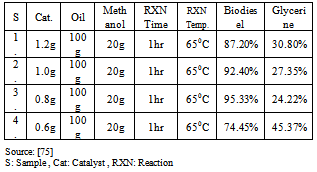
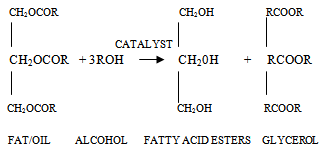 Several other side reaction occurs which if uncontrolled hampers conversion, product yield and quality[20]. Usually 3 parameters have effect on the trans-esterification, namely[33];• temperature (T), reaction• time (t) and • ratio of oil to alcohol.The reaction temperature plays an important role on the quality of the products. Kapilakarn K and .Peugtong[33] reported that normally, the range of the temperature used in the process is between. 50 0C – 65 oC. The temperature which is higher than the normal boiling point of methanol (68 oC) causes more vaporization of methanol (loss). On the other hand, the temperature which is lower than 50 oC causes higher viscosity of biodiesel[34].The ratio of methanol to oil also affects the reaction, the higher molar ratio, the higher conversion of alcohol. The ratios, normally used, are between 5:1 to 10:1[35]. However using too high excess methanol can obstruct glycerin separation[36]. Divya,B and Tyagi,V.K[20] investigated the conversion rate into biodiesel using beef tallow, sunflower , and soybean feed stocks. For beef tallow, reaction rate was very slow during the first minute due to the mixing and dispersion of methanol into beef tallow, the reaction proceeded very fast for the next five minutes. An approximate yield of 80 % was observed after 1 minute for soybean and sunflower oils at methanol to oil ratio of 6:1. After 1 hour, the conversions were almost the same (93%-98%).The effect of reaction time for palm oil at 40:1 methanol: oil with 5% H2SO4 (v/v) at 95 ℃ for 9 hours and obtained a maximum yield of 97 %[42]. However,further efforts is required to demonstrate the reactor ideal enough for biodiesel synthesis and its design and fabrication.
Several other side reaction occurs which if uncontrolled hampers conversion, product yield and quality[20]. Usually 3 parameters have effect on the trans-esterification, namely[33];• temperature (T), reaction• time (t) and • ratio of oil to alcohol.The reaction temperature plays an important role on the quality of the products. Kapilakarn K and .Peugtong[33] reported that normally, the range of the temperature used in the process is between. 50 0C – 65 oC. The temperature which is higher than the normal boiling point of methanol (68 oC) causes more vaporization of methanol (loss). On the other hand, the temperature which is lower than 50 oC causes higher viscosity of biodiesel[34].The ratio of methanol to oil also affects the reaction, the higher molar ratio, the higher conversion of alcohol. The ratios, normally used, are between 5:1 to 10:1[35]. However using too high excess methanol can obstruct glycerin separation[36]. Divya,B and Tyagi,V.K[20] investigated the conversion rate into biodiesel using beef tallow, sunflower , and soybean feed stocks. For beef tallow, reaction rate was very slow during the first minute due to the mixing and dispersion of methanol into beef tallow, the reaction proceeded very fast for the next five minutes. An approximate yield of 80 % was observed after 1 minute for soybean and sunflower oils at methanol to oil ratio of 6:1. After 1 hour, the conversions were almost the same (93%-98%).The effect of reaction time for palm oil at 40:1 methanol: oil with 5% H2SO4 (v/v) at 95 ℃ for 9 hours and obtained a maximum yield of 97 %[42]. However,further efforts is required to demonstrate the reactor ideal enough for biodiesel synthesis and its design and fabrication.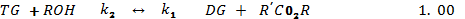
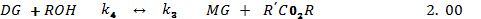
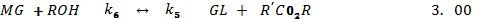
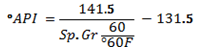 A low API gravity indicates heavier oil, while a higher API gravity means a lighter crude or product. Specific gravities of crude oils roughly range from 0.82 for lighter Crudes to over 1.0 for heavier oil.• Flash Point: This is the minimum temperature at which the vapour from oil sample will give a momentary flash on application of a standard flame under specific test conditions. This is used to predict the possible fire hazard during transportation, storage and handling.• Pour Point, Cloud Point: The pour point of a crude oil or product is the lowest temperature at which oil is observed to flow under the conditions of the test. Handling and transporting oils and heavy fuels is difficult at temperatures below their pour points .Often, chemical additives known as pour point depressants are used to improve the flow properties of the fuel. The temperature at which wax crystals begin to form on the surface of the biodiesel is the cloud point.• Aniline Point, Diesel Index: Aniline Point is the minimum temperature at which equal volumes of anhydrous aniline and oil mix together. A low aniline point indicates low diesel index. Diesel index is a measure of ignition quality of a fuel. Aniline point can also predict the amount of carbon present in the oil as given by the equation
A low API gravity indicates heavier oil, while a higher API gravity means a lighter crude or product. Specific gravities of crude oils roughly range from 0.82 for lighter Crudes to over 1.0 for heavier oil.• Flash Point: This is the minimum temperature at which the vapour from oil sample will give a momentary flash on application of a standard flame under specific test conditions. This is used to predict the possible fire hazard during transportation, storage and handling.• Pour Point, Cloud Point: The pour point of a crude oil or product is the lowest temperature at which oil is observed to flow under the conditions of the test. Handling and transporting oils and heavy fuels is difficult at temperatures below their pour points .Often, chemical additives known as pour point depressants are used to improve the flow properties of the fuel. The temperature at which wax crystals begin to form on the surface of the biodiesel is the cloud point.• Aniline Point, Diesel Index: Aniline Point is the minimum temperature at which equal volumes of anhydrous aniline and oil mix together. A low aniline point indicates low diesel index. Diesel index is a measure of ignition quality of a fuel. Aniline point can also predict the amount of carbon present in the oil as given by the equation 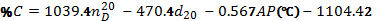 Where
Where 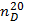 = Refractive index at 20 ℃ and
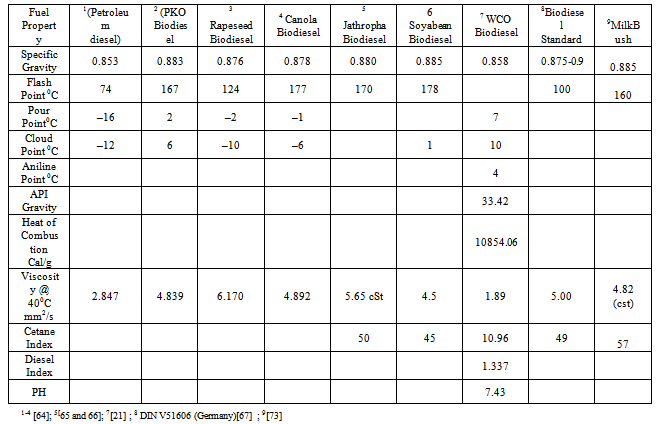
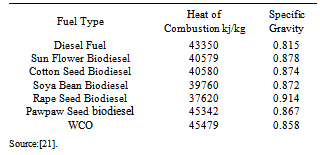
= Refractive index at 20 ℃ and 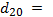 Density at 20℃.• Viscosity: This is the resistance to flow of oil. Ease of starting depends on viscosity. Glycerin contamination may cause biodiesel viscosity to increase[20].Production of biodiesel with much reduced viscosity and cloud point can be found in the patent of Nourreddini[68]. Tables 4-5 shows the fuel properties of biodiesel from various sources.
Density at 20℃.• Viscosity: This is the resistance to flow of oil. Ease of starting depends on viscosity. Glycerin contamination may cause biodiesel viscosity to increase[20].Production of biodiesel with much reduced viscosity and cloud point can be found in the patent of Nourreddini[68]. Tables 4-5 shows the fuel properties of biodiesel from various sources. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML