-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2012; 2(3): 78-85
doi: 10.5923/j.ijee.20120203.04
Performance Evaluation of Kiln for Cashew Nut Shell Carbonization and Liquid
S. H. Sengar , A. G. Mohod , Y. P. Khandetod
Department of Electrical and Other Energy Sources, College of Agricultural Engineering and Technology, DBSKKV, Dapoli
Correspondence to: A. G. Mohod , Department of Electrical and Other Energy Sources, College of Agricultural Engineering and Technology, DBSKKV, Dapoli.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Cashew nut shell (CNS) was utilized for carbonization in developed prototype kiln. Prototype kiln was evaluated with direct and indirect methods and characteristics of CNS and CNS char were determined by proximate and ultimate analysis. The maximum CNS temperatures obtained inside the kiln during direct and indirect method were recorded as 452.2℃ and 458.8℃ respectively. Maximum oil percentage, charcoal percentage and ash percentage in direct method were observed as 21.1 per cent, 21.04 per cent and 3.34 per cent respectively whereas 23.8 per cent, 18.3 per cent and 1.27 per cent in indirect method respectively. Hydrogen content in CNS was found about 6 to 7 per cent and nitrogen content in CNS was found about 0.70 to 0.75 per cent. Oxygen content in CNS was observed about 29 to 31 percent. Carbon, hydrogen and nitrogen content of the CNS char were observed in the range of 73 to 76 per cent, 4 to 5 per cent and 1 to 2 per cent respectively. It was found that nitrogen content has increased in CNS char after the carbonization of CNS. Oxygen content in the CNS char gets reduced to 13 to 14 percent, which was comparatively very less than CNS. It was observed that indirect method is more suitable for carbonization than direct method for obtaining higher calorific value char and maximum fixed carbon percentage as found in cashew nut shell char as 60 per cent.
Keywords: Cashew Nut Shell, Kiln, Char, CNSL
Article Outline
1. Introduction
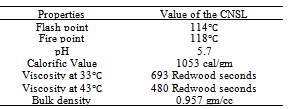
- In Konkan region the cashew (Anacardium occidentale L) is one of the major horticultural crops. Cashew is an important evergreen tropical crop. India is the largest producer, processor, exporter and second largest consumer of cashew in the world (http://www.cashewindia.org). Total area in India under cashew cultivation is about 8, 54,000 ha with annual production of 6, 20,000 tones giving average productivity 820 kg/ha with highest productivity reported in Maharashtra (1500 kg/ha) from 1,73,601 ha (Fig.1) under cultivation (Haldankar et al. 2007) and produced 1,97,000 tones of raw cashew nut seeds through 2200-3650 cashew processing units (2009). Production of Cashew nut shell in Koknan region is 20,000 metric tones (Mohod et al. 2010) as waste product obtained during deshelling of cashew kernels. When CNSL removed, deoiled shells are abundantly available as a biomass waste (Bisana and Laxamana 2008). The shell comprises some 50% of the weight of the raw nut, the kernel represents 25% and the remaining 25% consists of the natural CNSL (Rajapakse et al. 1977; Ramanan et al. 2008; Santos and Magalhães 1999) and shell production of cashew nut shells may be estimated to 3,10,000 tones from available stats. The cashew nut shell has calorific value of 4252 kcal/kg (Weihong and Blasiak 2006) and calorific value of CNSL is 40MJ/kg (Das and Ganesh 2003). The waste biomass generated in cashew processing is utilized as a substitute to wood fuel by making charcoal with carbonization process (Das et al. 2004). Biocarbons have been manufactured by man for more than 38, 00 years (Bard 2001) and are among the most important renewable fuels in use today.
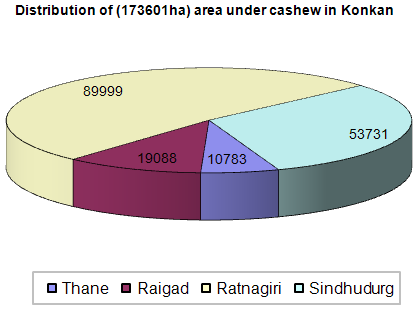 | Figure 1. Area under cashew cultivation in Konkan region of Maharashtra |
2. Material and Methods
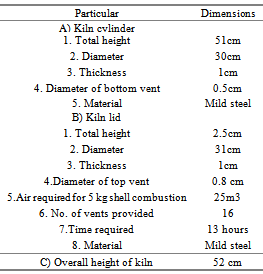
- The cashew nut shell available in the cashew processing industry was selected as a major raw material (Mohod et al. 2010) for the carbonization process. The proximate (Singh 2006; Ramanan et al. 2008) and ultimate analysis of CNS was carried out to find out the fuel properties. In addition to CNS properties, carbonization processes were studied using metal kiln and tin boxes under direct and indirect process.Experimental set up for carbonization of cashew nut shellThe carbonization of CNS was carried out in developed small capacity single drum kiln (Nienhuys I S 2003), which accommodate about 5 kg cashew nut shells. The kiln (Plate 1.a) was designed with 8 mm diameter holes for the perforations with outlet for the oil at the bottom and vent for the exhaust at the top ( Plate 1c). The size selected was suitable for small amount of burning, low cost and fabrication could be developed locally. Design detail specification of kiln is depicted in Table 1. At the beginning, the small vent at the top and the oil collecting tube at bottom allowed the burning of the cashew nut shells, and then operated as the pyrolysis mode in a closed kiln without air. Kiln was evaluated with and without perforated hole. The selected small capacity kiln was used for direct carbonization process (Plate 1.a). The weight of raw CNS was measured and filled the kiln with full capacity and burn directly. Various temperatures at different location under kiln, exhaust temperature of vent and oil collected from the bottom sump were observed periodically. Time require to complete carbonization process and amount of char remain in kiln was observed at the end of process. The detailed flow chart of carbonization processed used is shown in Fig.2.
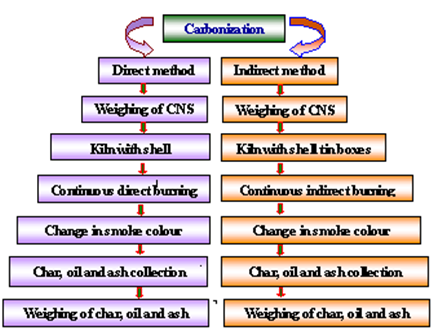 | Figure 2. Process flow chart of carbonization methods |
|
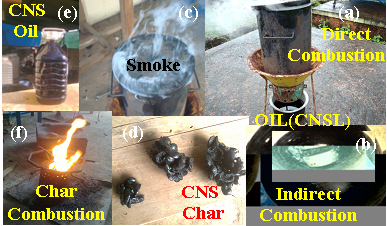 | Plate 1. Prototype kiln for carbonization |
3. Proximate analysis of CNS and its Char
- Determination of moisture contentAbout 1g of finely powdered air-dried sample was weighed in a crucible. The crucible was placed inside an electric hot air-oven, maintained at 105℃. The crucible was allowed to remain in oven for 1 hour and then taken out (with the help of a pair of tongs), cooled in desiccators and weighed. Loss in weight (ASTMD-3173) was reported as moisture (on percentage-basis). (Dara 1999)Moisture content, (% wb) =
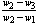 × 100Where, w1 = weight of crucible, gw2 = weight of crucible + sample, gw3 = weight of crucible + sample, after heating, gDetermination of volatile matter volatile matterVolatile matter was determined by keeping the dried sample in a closed crucible at 600℃ for six minutes and then at 900℃ for another six minutes (ASTMD-3275). The difference in the weight duo to loss of volatiles was taken as the total volatile matter present in the biomass. Loss in weight was reported as volatile matter on percentage basis.Volatile matter (%) =
× 100Where, w1 = weight of crucible, gw2 = weight of crucible + sample, gw3 = weight of crucible + sample, after heating, gDetermination of volatile matter volatile matterVolatile matter was determined by keeping the dried sample in a closed crucible at 600℃ for six minutes and then at 900℃ for another six minutes (ASTMD-3275). The difference in the weight duo to loss of volatiles was taken as the total volatile matter present in the biomass. Loss in weight was reported as volatile matter on percentage basis.Volatile matter (%) = 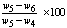 Where,w4 = weight of crucible + weight of sample before oven drying, gw5 = weight of crucible + weight of sample before keeping in muffle furnace, gw6 = weight of crucible + weight of sample after keeping in muffle furnace, gDetermination of ash content:The residual coal in the crucible in (2) was then heated without lid in a muffle furnace at 750 ℃ for half hour for (ASTMD-3174). The crucible was then taken out, cooled first in air, then in desiccators and weighed. Heating, cooling and weighing was repeated, till a constant weight was obtained. The residue was reported as ash on percentage-basis.Ash content, (%) =
Where,w4 = weight of crucible + weight of sample before oven drying, gw5 = weight of crucible + weight of sample before keeping in muffle furnace, gw6 = weight of crucible + weight of sample after keeping in muffle furnace, gDetermination of ash content:The residual coal in the crucible in (2) was then heated without lid in a muffle furnace at 750 ℃ for half hour for (ASTMD-3174). The crucible was then taken out, cooled first in air, then in desiccators and weighed. Heating, cooling and weighing was repeated, till a constant weight was obtained. The residue was reported as ash on percentage-basis.Ash content, (%) = 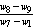 × 100Where,w7 = weight of crucible + weight of sample before oven drying, gw8 = weight of crucible + weight of sample before keeping in muffle furnace, gw9 = weight of crucible + weight of sample after keeping in muffle furnace, gDetermination of fixed carbon:The fixed carbon in percentage was calculated by difference.Fixed carbon (%) = 100 – % of (moisture content + volatile matter +ash)Procedure for determination of calorific value of the fuel by using the Oxygen Bomb CalorimeterA known mass of the given sample was taken in clean crucible. The crucible was then supported over the ring. A fine magnesium wire, touching the fuel sample, was then stretched across the electrodes. The bomb lid was tightly screwed and bomb filled with oxygen to 25 atmospheric pressure. The bomb was then lowered into copper calorimeter, containing a known mass of water. The stirrer was worked and initial temperature of the water was noted. The electrodes are then connected to 6-volt battery and circuit completed. The sample burns and heat was liberated. Uniform stirring of water was continued and the maximum temperature attained was recorded, the experimental setup for determination of calorific value using Bomb calorimeter.The calorific value of the CNS and its char was determined by using Bomb Calorimeter. The calorific value of the CNS and its char was determined by using the following formula (Dara 1999).Calorific value (kcal/kg) =
× 100Where,w7 = weight of crucible + weight of sample before oven drying, gw8 = weight of crucible + weight of sample before keeping in muffle furnace, gw9 = weight of crucible + weight of sample after keeping in muffle furnace, gDetermination of fixed carbon:The fixed carbon in percentage was calculated by difference.Fixed carbon (%) = 100 – % of (moisture content + volatile matter +ash)Procedure for determination of calorific value of the fuel by using the Oxygen Bomb CalorimeterA known mass of the given sample was taken in clean crucible. The crucible was then supported over the ring. A fine magnesium wire, touching the fuel sample, was then stretched across the electrodes. The bomb lid was tightly screwed and bomb filled with oxygen to 25 atmospheric pressure. The bomb was then lowered into copper calorimeter, containing a known mass of water. The stirrer was worked and initial temperature of the water was noted. The electrodes are then connected to 6-volt battery and circuit completed. The sample burns and heat was liberated. Uniform stirring of water was continued and the maximum temperature attained was recorded, the experimental setup for determination of calorific value using Bomb calorimeter.The calorific value of the CNS and its char was determined by using Bomb Calorimeter. The calorific value of the CNS and its char was determined by using the following formula (Dara 1999).Calorific value (kcal/kg) = 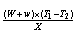 Where, W = weight of water in calorimeter (kg),w = water equivalent of apparatus,T1 = initial temperature of water (℃),T2 = final temperature of water (℃), X = weight of fuel sample taken (kg)Ultimate analysis of CNS and charCarbon, hydrogen, oxygen, nitrogen, and sulphur content of CNS and its char were found out under the ultimate analysis. The ultimate analysis is helpful in calculating heat balances in any process in which coal is used as fuel. Using the values of proximate analysis, ultimate analysis of CNS char could be done theoretically by using the various formula.Calculation of C, H, N and O from the proximate analysisThese values were carried out through the following steps Determination of carbon contentCarbon content of the sample was calculated theoretically on the basis of following formula,C = 0.97 FC + 0.7 (VM – 0.1 A) – M (0.6 – 0.01 M), %Determination of hydrogen contentHydrogen content of the sample was calculated theoretically which was given by,H = 0.036 FC + 0.086 (VM – 0.1 A) – 0.0035 M2 (1 – 0.02 M), %Determination of nitrogen contentNitrogen content of the sample was calculated theoretically which was given by,N2 = 2.10 – 0.020 VM, %Determination of oxygen contentOxygen content of the sample was calculated theoretically by difference on the basis of the following formula,O2 = 100 - % of (C + H + N + Ash), %Where, FC = fixed carbon, %A = ash, %VM = volatile matter, %M = moisture, %Results and DiscussionsCNS was selected as a biomass for carbonization because CNS was treated as waste in Konkan region and it is abundantly available in this region. Bulk density of CNS was 481.83 kg/m3, calorific value was nearly about 5000 kcal/kg and oil content was about 20 to 25 percent. The calorific values and oil content values were observed similar to values observed by Ramanan et al. 2008.Carbonization of CNSIn the carbonization process, solid residues with increasing content of the element carbon were formed from the organic material usually by pyrolysis in an inert atmosphere. Char preparation by this method would help in self reliance of cashew processing unit for fuel supply with higher combustion efficiency. The carbonization process of CNS was carried out in direct and indirect ways, which results into the production of charcoal (Plate 1 d) as the main product plus gaseous product along with the CNSL (Plate 1 e) and ash. For thorough study of the carbonization process, shell temperature inside the kiln, exhaust temperature at the top vent and the outside temperature of the cylinder were recorded. The temperatures recorded in the direct and indirect method are shown below in the graphical format in Fig. 3 and Fig. 4 respectively.It was observed from the Fig. 3 and Fig. 4 that time required to reach maximum temperature inside the kiln using indirect method was comparatively less than direct method for obtaining CNS char. maximum temperature was achieved in indirect method in less time while direct method took more time for the carbonization. This may happen due as temperature of dried fuel was evaluated to about 225-325 ℃, pyrolysis of hemicelluloses begins, cellulose gets pyrolysed at a temperature range of 325 ℃ –375 ℃ while legnin starts pyrolysing at a temperature range of 350-500 ℃ (Shafizadeh and Chin 1997). Pyrolysis gases escape and char layer is form on fuel particle. It could be observed, in both methods, inside temperature varies more drastically than outside exhaust temperatures. Average temperature of shell at core part of kiln varies from 86 ℃ to 445℃ in direct method whereas it varies from 80℃ to 448℃ in indirect method. Average exhaust temperature at the top vent varies from 172℃ to 218℃ in direct method while it varies from 175℃ to 230℃ in indirect method. Average outside temperature of the kiln was recorded in the range of 44℃ to 60℃ for direct method and 450 to 61℃ for indirect method. It was also observed that smoke colour gradually changes from blackish to white and then from white to colourless when all CNS turned to charcoal at the temperature 350℃ onwards in both the methods.
Where, W = weight of water in calorimeter (kg),w = water equivalent of apparatus,T1 = initial temperature of water (℃),T2 = final temperature of water (℃), X = weight of fuel sample taken (kg)Ultimate analysis of CNS and charCarbon, hydrogen, oxygen, nitrogen, and sulphur content of CNS and its char were found out under the ultimate analysis. The ultimate analysis is helpful in calculating heat balances in any process in which coal is used as fuel. Using the values of proximate analysis, ultimate analysis of CNS char could be done theoretically by using the various formula.Calculation of C, H, N and O from the proximate analysisThese values were carried out through the following steps Determination of carbon contentCarbon content of the sample was calculated theoretically on the basis of following formula,C = 0.97 FC + 0.7 (VM – 0.1 A) – M (0.6 – 0.01 M), %Determination of hydrogen contentHydrogen content of the sample was calculated theoretically which was given by,H = 0.036 FC + 0.086 (VM – 0.1 A) – 0.0035 M2 (1 – 0.02 M), %Determination of nitrogen contentNitrogen content of the sample was calculated theoretically which was given by,N2 = 2.10 – 0.020 VM, %Determination of oxygen contentOxygen content of the sample was calculated theoretically by difference on the basis of the following formula,O2 = 100 - % of (C + H + N + Ash), %Where, FC = fixed carbon, %A = ash, %VM = volatile matter, %M = moisture, %Results and DiscussionsCNS was selected as a biomass for carbonization because CNS was treated as waste in Konkan region and it is abundantly available in this region. Bulk density of CNS was 481.83 kg/m3, calorific value was nearly about 5000 kcal/kg and oil content was about 20 to 25 percent. The calorific values and oil content values were observed similar to values observed by Ramanan et al. 2008.Carbonization of CNSIn the carbonization process, solid residues with increasing content of the element carbon were formed from the organic material usually by pyrolysis in an inert atmosphere. Char preparation by this method would help in self reliance of cashew processing unit for fuel supply with higher combustion efficiency. The carbonization process of CNS was carried out in direct and indirect ways, which results into the production of charcoal (Plate 1 d) as the main product plus gaseous product along with the CNSL (Plate 1 e) and ash. For thorough study of the carbonization process, shell temperature inside the kiln, exhaust temperature at the top vent and the outside temperature of the cylinder were recorded. The temperatures recorded in the direct and indirect method are shown below in the graphical format in Fig. 3 and Fig. 4 respectively.It was observed from the Fig. 3 and Fig. 4 that time required to reach maximum temperature inside the kiln using indirect method was comparatively less than direct method for obtaining CNS char. maximum temperature was achieved in indirect method in less time while direct method took more time for the carbonization. This may happen due as temperature of dried fuel was evaluated to about 225-325 ℃, pyrolysis of hemicelluloses begins, cellulose gets pyrolysed at a temperature range of 325 ℃ –375 ℃ while legnin starts pyrolysing at a temperature range of 350-500 ℃ (Shafizadeh and Chin 1997). Pyrolysis gases escape and char layer is form on fuel particle. It could be observed, in both methods, inside temperature varies more drastically than outside exhaust temperatures. Average temperature of shell at core part of kiln varies from 86 ℃ to 445℃ in direct method whereas it varies from 80℃ to 448℃ in indirect method. Average exhaust temperature at the top vent varies from 172℃ to 218℃ in direct method while it varies from 175℃ to 230℃ in indirect method. Average outside temperature of the kiln was recorded in the range of 44℃ to 60℃ for direct method and 450 to 61℃ for indirect method. It was also observed that smoke colour gradually changes from blackish to white and then from white to colourless when all CNS turned to charcoal at the temperature 350℃ onwards in both the methods.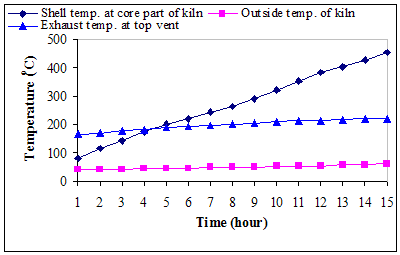 | Figure 3. Variation of carbonization temperature with time during direct method |
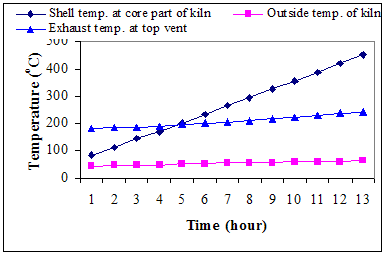 | Figure 4. Variation of carbonization temperature with time during indirect method |
|
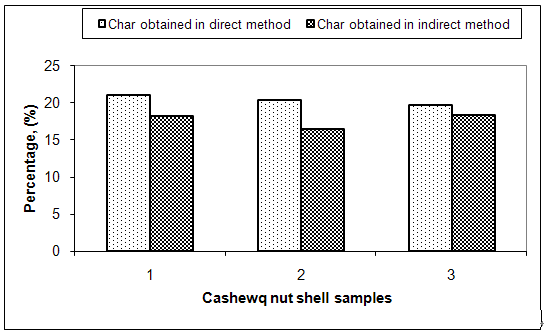 | Figure 5. Comparison of direct and indirect method for char percentage |
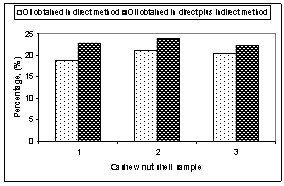 | Figure 6. Comparison of direct and direct plus indirect method for oil percentage |
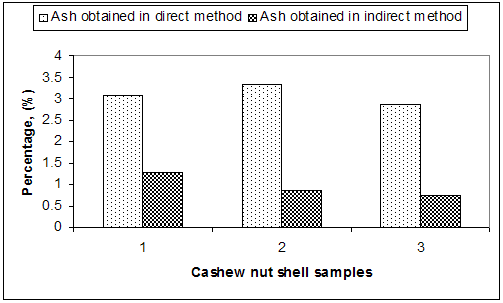 | Figure 7. Comparison of direct and indirect method for ash percentage |
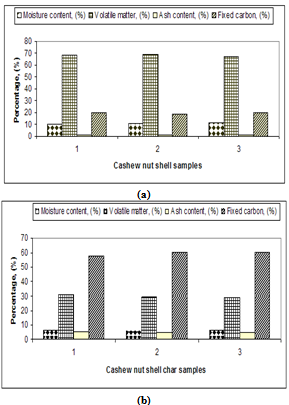 | Figure 8. Proximate analysis of cashew nut shell and its char |
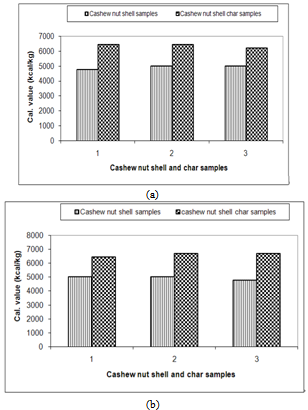 | Figure 9. Calorific value of cashew nut shell and its char |
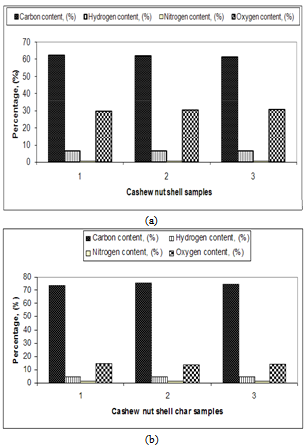 | Figure 10. Ultimate analysis of CNS and its char |
4. Conclusions
- 1. It was observed that indirect method is more suitable for carbonization than direct method for higher calorific value char.2. It was observed that time required for the carbonization using indirect method was comparatively less than direct method.3. It was observed that maximum fixed carbon percentage found in cashew nut shell char as 60 per cent.
Acknowledgements
- Authors are highly thankful to Department of Electrical and Other Energy Sources, College of Agricultural Engineering and Technology, DBSKKV, Dapoli for giving the facility to carry out research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML