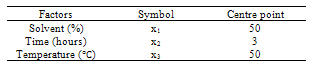-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Energy Engineering
p-ISSN: 2163-1891 e-ISSN: 2163-1905
2012; 2(2): 8-14
doi: 10.5923/j.ijee.20120202.02
Nonlinear Programming for Solvent Extraction of Jatropha Curcas Seed Oil for Biodesiel Production
O. O. Ogunleye 1, O. A. A. Eletta 2
1Department of Chemical Engineering, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Nigeria
2Department of Chemical Engineering, University of Ilorin, P.M.B. 1515, Ilorin, Nigeria
Correspondence to: O. O. Ogunleye , Department of Chemical Engineering, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Nonlinear programming models of solvent extraction of Jatropha curcas oil that forms the thrust of this paper is uncommon in literature. The oil was extracted using two solvents (n-hexane and Isopropanol) at a powder weight to solvent volume of 1:5 and particle size of between 0.5mm and 0.75 mm. A randomized 31 set of central composite design comprising three factors (solvent composition[0 - 100% n-haxane], time of extraction[1 - 5hours] and extraction temperatures[40 – 60℃) at five levels were experimented. Oil yield, specific gravity, viscosity, free fatty acid (FFAs) and iodine value of the oil extracts were determined. Response equation of each of oil yield (R1), specific gravity (R2), viscosity (R3), FFA (R4) and iodine value (R5) in terms of solvent composition (X1), time of extraction (X2) and temperature (X3) were developed. These were formulated into a nonlinear programme that maximize oil yield and minimizes other four properties according to ASTM D6751-07b and EN 14214-2008 (E) standards for biodiesel production. The coefficients of determination (R2) for the responses equations were 1.000, 1.000, 0.953, 0.963 and 0.968 respectively. The nonlinear programme yields a maximum oil yield (R1) of 37.3507 %, R2 = 0.88597, R3 =39.771 , R4 = 2.1185 and R5 = 101.51, while the optimum operating conditions were X1 = 2 (100% n haxane), X2 =2 (5 hours ) and X3 = 2 (60℃). This study has clearly demonstrated the applicability of nonlinear mathematical programming in selecting extraction conditions for jatropha oil from its seed.
Keywords: Jatropha Oil , Oil Yield, Nonlinear, Biodiesel, Optimum
Article Outline
1. Introduction
- Jatropha curcas belongs to a very large family, Euphorbiaceae. It has numerous common names depending on the country where it is found, but is most commonly referred to as physic nut or purging nut[1]. Jatropha curcas is known in Nigeria by various local names as “butuje”, “lapapa”, “ologbo”[2]. The seeds of Jatropha contain viscous oil, which can be used for the manufacture of candles and soap, in the cosmetics industry, as a diesel/paraffin substitute or extender that is a practical substitute for fossil fuels to counter greenhouse gas accumulation in the atmosphere[3]. It has a yield per hectare of more than four times that of soya beans and 10 times that of corn[4]Jatropha oil belongs to the family of vegetable oils that are now promising alternatives in solving energy problems due to several advantages; it is renewable, environ-friendly and can be produced easily in rural areas, where there is an acute need for modern forms of energy[5]. Many of these oils, including palm oil, soybean oil, sunflower oil, rapeseed oil, and canola oil have been used to produce biodiesel fuel and lubricants[5]. The fact that Jatro- pha oil cannot be used for nutritional purposes without detoxification makes its use as fuel source very attractive.Several methods can be used to obtain vegetable oil from the seeds; this include, mechanical pressing, supercritical fluid extraction, and solvent extraction[6]. Mechanical extraction is the most widely used method of extraction. However, the oil produced with this method is turbid, contains a significant amount of water, and metals contents, which makes them less suitable for biodiesel production[7]. In extraction using supercritical fluid, the oil produced has very high purity; however, the operating and investment cost is high. Extraction using solvent has several advantages, which includes; higher yield and less turbid oil than mechanical extraction, and relative low operating cost compared with supercritical fluid extraction.The use of vegetable oil in producing fuel is limited to diesel engine because they contain free fatty acids (FFAs), phospholipids, sterols and other impurities[7]. They have very high viscosity which is about 17 times higher than that of the fossil fuel and can affect spray atomization, vapourization and air –fuel mixing in the combustion chambers, leading to an adverse effect on the combustion chamber[8]. Production of biodiesel from Jatropha oil has been studied by several researchers with different production processes and conditions[9-15].Biodiesel is a fuel obtained from renewable biomass feedstock (It is renewable, non-toxic and biodegradable) which consists of mono alkyl esters usually produced by transeterification of vegetable oils, fat and cooking oil using low molecular weight alcohols. Commonly used alcohols are methanol and ethanol[16]. Transesterification can be carried out with or without a catalyst and using a base or acid catalyst. Studies have reported greater yield using an acid catalyst. When NaOH was used, at the optimized conditions, biodiesel yield was only 47.2 % and soap foams were formed when product was dropped in distilled water, which solidifies to soap like material within 2 hours in a saponification reaction between NaOH and FFA. Transesterification of Jatropha oil using super critical methanol in the absence of catalyst has been studied[17]. With H2SO4 as catalyst, and at a longer reaction time, biodiesel yield was increased to 92.8%. FFA in bio diesel can be reduced using a two-step approach with potassium hydroxide as a catalyst[18].The crux of the transesterification for biodiesel production remains that of FFA, moisture contents, viscosity, iodine value and saponification value of the Jatropha oil feedstock. High FFA above 1% w/w yields low biodiesel production but favours the production of soap. This low FFA is achieved through mechanical extraction but fails the moisture content requirement that is more critical. High viscosity and iodine value of Jatropha oil are not desirable for biodiesel production for operational reasons like carbon deposits and oil ring sticking[5]. However, the yield of extractable oil from Jatropha and its properties have been said to depend on the composition of the extract solvent, the temperature and the time of heating[19-20].In this study, solvent extraction of oil from Jatropha seed was studied to investigate the effects of process parameter such as temperature, time and solvent composition on the yield and quality of the extract. This also led to the formation and solution of nonlinear programming model for maximizing oil yield subject to biodiesel feedstock requirements.
2. Materials and Method
2.1. Materials Preparation
- Jatropha curcas seed used in this study were obtained from , Nigeria. The seed had moisture content of 6.20% and oil content of 40.0%[21]. The seeds were repeatedly washed to remove dirts and other impurities. It was subsequently dried in oven at 50℃ until it reached constant moisture content. These seeds were grounded and sieved to get particle size of between 0.5mm and 0.75 mm.
2.2. Oil Extraction
- Jatropha curcas seed oil was extracted using two solvents (n-hexane and Isopropanol). Jatropha seed powder weight (g) to solvent volume (ml) of 1:5 was adopted[6]. These extractions involved three factors (solvent composition, time of extraction and extraction temperature) at five levels as presented in the experimental design. At the set time interval, the samples were taken and centrifuged to separate the solid fraction from the solution. The extracts were heated and evaporated using rotary evaporator apparatus to obtain solvent-free oils.
2.3. Determination of Oil Properties
- The oil yield, specific gravity, viscosity, free fatty acid and iodine value were determined. Parameters were determined in triplicates and the mean taken for grater accuracy.(i) Oil Yield (%): The weight of oil extracted from 20 g of seed powder was determined and expressed as the percentage of oil in the dry matter of seed powders.(ii) Specific Gravity: The specific gravity of the samples was determined at 25℃.(iii) Viscosity (centipoises): Viscosity of seed oil was carried out using rotational viscometer at 10 rpm at a temperature 25℃.(iv) Free Fatty Acid (FFA), (%): Acid value of seed oil was determined according to AOAC Official Method Cd 3a- 63. Percentage free fatty acids (FFAs) were calculated using oleic acid as a factor.(v) Iodine value (g iodine /100 g): Iodine value of seed oil was determined according to AOAC Official Method 993.20.
2.4. Experimental design
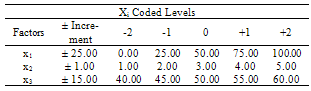
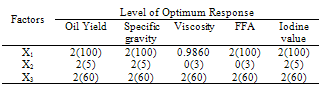
- The design was based on the fact that oil yields and physical properties are functionally related to the three factors (solvent composition, time of extraction and extraction temperature)[22]. A centre point for the design was selected with ingredients at levels expected to yield, at least, satisfactory experimental results. The list of the factors for centre point is as shown on Table 1.
|
|
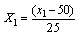 | (1) |
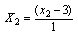 | (2) |
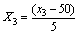 | (3) |
2.5. Response Equations
- As pointed out by[23] and[24], regression analysis provides a conceptually simple method for investigating functional relationships among variables. Regression models were formulated for each of the five properties of oil as a function of the three process factors. SPSS version 11.0 package was used for this analysis. In multiple regression as in the present case, R2, which is the square of the adjusted coefficient of determination and standard error are the indices. F statistics shows the significance of the overall model while the t statistics tests the significance of each of the variables of the model. The function was assumed to be approximated by a second degree polynomial equation :
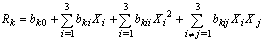 | (4) |
2.6. Nonlinear Programme for Oil Yield
- A nonlinear programming problem of the form of equations 5 to 10 was formed from the vector of equation 4 as follows:
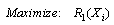 | (5) |
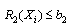 | (6) |
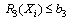 | (7) |
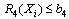 | (8) |
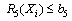 | (9) |
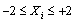 | (10) |
3. Results and Discussion
3.1. Response Equations
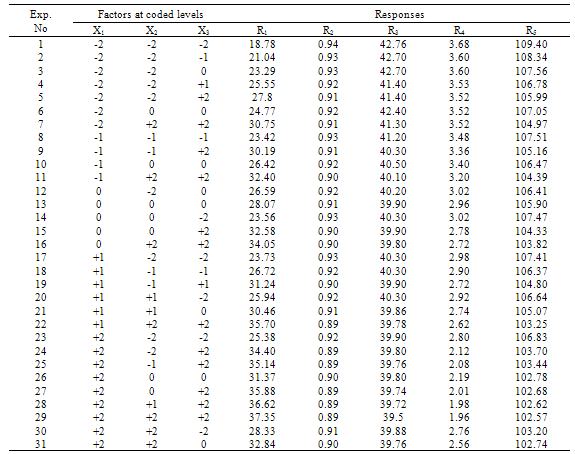
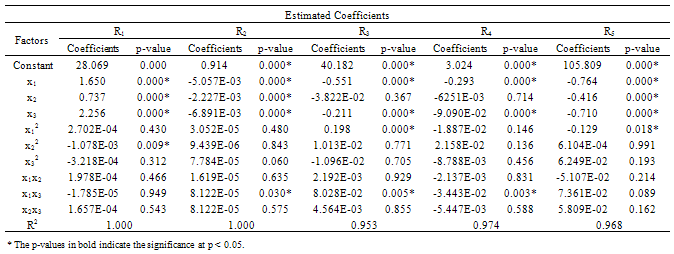
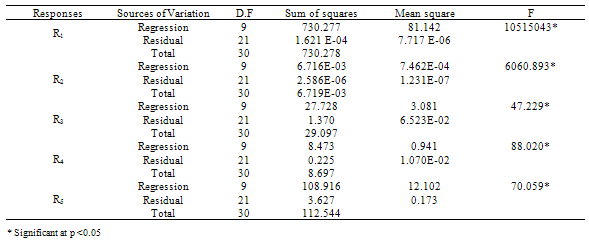
- The results of the fractional central composite design for evaluating the effects of solvent composition, time and temperature of extraction on the oil yield, density, viscosity, FFAs and Iodine values are presented in Table 3. This was subsequently used to fit the response equations for all the five oil properties. Table 4 shows the factors of the models, their parameter estimates and the statistics of the estimates for the best functions adopted, taking into consideration all main effects, linear, quadratic, and interaction for each model. Analyses were conducted to evaluate the adequacy and consistency of the models. The analysis of variance of the models is presented in Table 5. The analysis of variance calculated assessed how well the model represented the data. As shown on the Table 5, the F-value for the Oil yield is 10515043 that is significant at 95% level implying good model fit. Similar significant values were also evaluated for specific gravity, viscosity, FFA and Iodine value on Table 5. The coefficients of determination (R2) for the responses (1.000, 1.000, 0.953, 0.963 and 0.968 respectively) are quite high for response surfaces, and indicated that the fitted quadratic models accounted for more than 96% of the variance in the experimental data, which were found to be highly significant. Based on t-statistics, the only regression coefficients significant at 95 % were selected for inclusion in the equations (11) to (15).
|
|
|
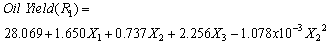 | (11) |
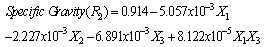 | (12) |
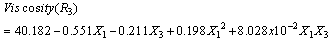 | (13) |
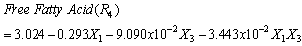 | (14) |
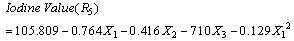 | (15) |
3.2. Optimum surface conditions for Jatropha Oil Extraction
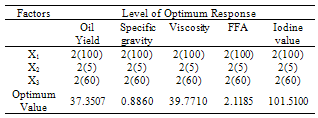
- The models (R1, R2, R3, R4 and R5) were useful for indicating the direction in which to change variables in order to maximize oil yield and minimize specific gravity, viscosity, FFA and Iodine values. The multiple regression equations were solved for the maximum oil yield (37.3507%), and minimum specific gravity (0.8860), viscosity (39.5675cp), FFA (2.1185 g/g) and Iodine value (101.5130). The optimum operating condition (coded) predicted for each corresponding response are as shown in Table 6, and are within the experimental range, indicating the validity of the selection of the variables range. The actual values are in the bracket. The response surfaces and contour graphs were obtained by plotting two variables, with the one remaining having the value, which give the optimum.
|
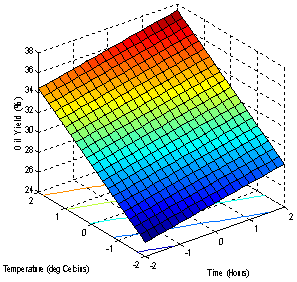 | Figure 1. Effect of factors on the Oil Yield |
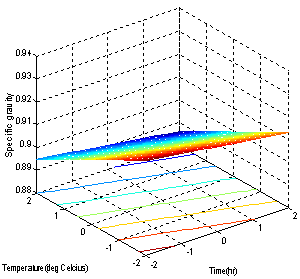 | Figure 2. Effect of factors on the Specific gravity |
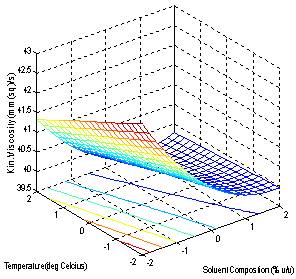 | Figure 3. Effect of Factors on the Viscosity |
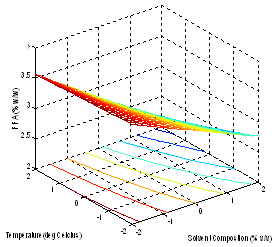 | Figure 4. Effect of factors on the FFA |
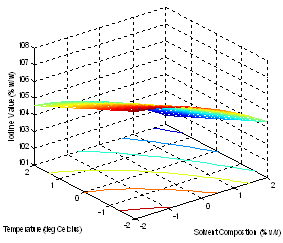 | Figure 5. Effect of factors on the Iodine Value |
3.3. Optimum Extraction for Biodiesel Production
4. Conclusions
- This study has clearly demonstrated the applicability of nonlinear mathematical programming in selecting extraction conditions for jatropha oil from its seed. This approach has not only resulted in the maximum oil yield through solvent extraction, but has also guaranteed the fulfillment of the requirements for using this oil as a feedstock for biodiesel production. Through this method, 37.35% oil yield has been achieved with specific gravity 0.8860, viscosity 39.7710 cp, FFA of 2.1185 % and Iodine value of 101.51 g/g. Such oil obtained from this method will not need expensive pretreatment for biodesel production as compared with other means.
ACKNOWLEDGEMENTS
- The technical support of the technologists at Chemical Engineering Analytical Laboratory, LAUTECH, Ogbomoso is acknowledged
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML