-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2017; 7(1): 17-20
doi:10.5923/j.ije.20170701.03

Impact of Psidium cattleianum Invasion on Soil Microbial Functioning and on Uapaca ferruginea (Baill.) Regeneration at Forest Edge in the Eastern Part of Madagascar
Rajaonarimamy E. 1, Rakotomalala J. Y. 2, Rakotoarimanana R. A. 3, Rajaonarison J. L. 3, Rakotozafy J. C. R. 3, Randriambanona H. 4, Ramanankierana H. 4, Robin D. 5, Andrianarisoa B. 1
1Laboratory of Biotechnology and Microbiology, Faculty of Sciences, University of Antananarivo - Madagascar
2Faculty of Sciences, University of Antananarivo Madagascar
3Laboratory of Molecular Biology, University of Fianarantsoa - Madagascar
4Laboratory of Environment Biology, CNRE, Antananarivo - Madagascar
5Laboratoire des Symbioses Tropicales et Méditerranéennes, UMR 113, CIRAD, IRD, Université de Montpellier 2, France
Correspondence to: Rakotozafy J. C. R. , Laboratory of Molecular Biology, University of Fianarantsoa - Madagascar.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Perturbations caused by alien species on native plant regeneration and on soil microbial dynamics are major problem to biodiversity conservation. In this study, our main objective was to evaluate the impact of the propagation of an exotic plant species, Psidium cattleianum, i) on soil Arbuscular Mycorrhizal (AM) fungus dynamics, and ii) on the regeneration of Uapaca ferruginea, a native species in the Eastern forest of Madagascar. Soil samples were collected within (i) a homogenous formation of P. cattleianum, (ii) a natural forest devoid of P. cattleianum dominated mainly by U. ferruginea, and (iii) a degraded soil outside the forest and devoid of P. cattleianum. Soil enzymatic activities, soil microbial activity, the Most Probable Number (MPN) of mycorrhizal propagules in soil and the total number of Arbucular Mycorrhiza spores in soil were assessed. Seedling development of U. ferruginea planted on these three soil samples were assessed after 5 months culturing under greenhouse conditions. The total numbers of AM Fungal spores were significantly high on colonized soil by P. cattleianum. Global microbial activity was significantly higher on natural forest soil than those recorded on the other soil samples. Seedling development of U. ferruginea decreased from soil forest to invaded soil by P. cattleianum and degraded soil.
Keywords: Biological invasion, Psidium cattleianum, Native essence, Regeneration, Uapaca ferruginea, Madagascar
Cite this paper: Rajaonarimamy E. , Rakotomalala J. Y. , Rakotoarimanana R. A. , Rajaonarison J. L. , Rakotozafy J. C. R. , Randriambanona H. , Ramanankierana H. , Robin D. , Andrianarisoa B. , Impact of Psidium cattleianum Invasion on Soil Microbial Functioning and on Uapaca ferruginea (Baill.) Regeneration at Forest Edge in the Eastern Part of Madagascar, International Journal of Ecosystem, Vol. 7 No. 1, 2017, pp. 17-20. doi: 10.5923/j.ije.20170701.03.
Article Outline
1. Introduction
- The fragility of island ecosystems in respect to the spread of alien exotic plants currently deserves careful consideration. Indeed, after the destruction of ecological habitats, the introduction of alien species is now regarded as the second direct reason of imbalance in the plant structure in the world [1]. Thus, the intensification of national and international trade of plants because of their socio-economical, ornamental or food value, has promoted the introduction of alien plant species in many countries in the world [1, 2].Propagation of these alien species disturbs some soil parameters affecting the development of native species such as mycorrhizal fungi functioning and structure, soil enzymatic activities and disturbing the conservation of biodiversity [3]. Different biological mechanisms are responsible of these disturbances: (i) modification of physico-chemical properties and microbial structure and functioning of soil through rhizodeposition or litter effect and (ii) competition with native species to acquire nutrients or light [4-6]. Indeed, some alien species caused more or less important changes at ground level, creating unfavorable condition to the establishment of native species [7, 8]. Thus, these species represent a serious threat to biodiversity as they can completely replace native species by invading the surroundings where they have been introduced [9]. In the eastern part of Madagascar, a rapid establishment of Psidium cattleianum on degraded natural forests was observed during last decade. At the same time, surveys showed that the number of native plant species decreased significantly around the invaded areas by Psidium cattleianum, (Ramanankierana, unpublished data). In this coastal forest, U. ferruginea constitutes one of the valuable woody species. The main objective of this study was to assess the impact of P. cattleianum propagation on mycorrhizal fungi community associated with U. ferruginea and on the seedling development of this tree species in the eastern forest ecosystem of Madagascar.
2. Materials and Methods
2.1. Study Site Description
- The study area is located in the forest ecosystem of Ianjomara-Vatomandry (S 19°07’, E 48°54’, 113 m), situated about 200 km east of Antananarivo, Madagascar. This forest represents one of the remaining forest fragments in this part of the island. Plots of 10 X 10 m have been materialized in this forest formation to obtain three microhabitats on the basis of plant composition: (i) natural forest dominated by U. ferruginea and devoid of P. cattleianum; (ii) forest edge with propagation of P. cattleianum (iii) degraded soil located outside the forest and devoid of both U. ferruginea and P. cattleianum and where vegetation consists mainly of herbaceous species. Three plots were established for each soil type. Soil samples from each plot were used to assess structure of mycorrhizal fungi communities, global microbial activities and soil enzymatic activities. They were prepared in the greenhouse for the plantation of U. ferruginea.
2.2. Assessment of AM Fungus Community Structure
- Density and morphological diversity of arbuscular mycorrhizal fungi spores were determined using the method described by Sieverding et al. [10]. AM fungal spores were extracted from soil samples by wet sieving and decanting, followed by sucrose centrifugation [11]. After centrifugation, the supernatant was poured through a 50-mm sieve and rinsed with tap water. Spores were counted under a stereomicroscope and grouped according to morphological characteristics. The relative abundance of each fungal type was calculated per 100g of dry soil. Spore size and color were assessed in water under a stereomicroscope (Olympus SZ H10 research stereomicroscope) whereas spore wall structures and other attributes were observed on permanent slides prepared according to Azcon-Aguilar et al. [12] under a microscope connected to a computer with digital image analysis software. For enumerating infective propagules of vesicular arbuscular mycorrhizal fungi in soil, the MPN was determined using the method described by Porter et al. in [13].
2.3. Measurement of pH and Soil Microbial Functionalities
- For each type of soil, pH of a water soil suspension was determined. Total microbial activity in soil samples was measured using the fluorescein diacetate (3’, 6’,-diacetylfluorescein [FDA]) hydrolysis assay according to the method of Alef et al. in [14] in which fluorescein liberated was assayed colorimetrically at 490 nm, after 1 h of soil incubation. Soil phosphatase activity was measured by absorbance reading at 400 nm following the method of [15] with p-Nitrophenyl Phosphate (pNPP) as an artificial electron acceptor to form para nitrophenol (pN) under acid and alkaline condition. Result was expressed as the released para nitrophenol per g of soil per hour.
2.4. Seedling Development of U. ferruginea
- Seeds of U. ferruginea were surface sterilized with 70% of ethanol for 5 min. They were pre-germinated for 5 days in sterilized sands humidified with distillated water at 25°C in the dark. For each soil origin, plastic pots (4 cm diameter, 20 cm height) were filled with 250 g of soil and one pre-germinated seed of U. ferruginea was planted per pot. 50 replicates per treatment were established and pots were arranged in a randomized complete block design in the greenhouse and seedlings grown under natural light (daylight of approximately 12 h, average daily temperature of 25°C). They were watered regularly with tap water without fertilizer.After 5 months of cultivation, measurement of the plants heights was made. Then the plants were harvested and the oven-dried weight (1 week at 65°C) of shoot was measured. Their entire root systems were washed under tap water. On each plant, the extent of AM colonization was assessed (Phillips & Hayman, 1970) and the total dry weight of root per plant (1 week at 65°C) determined.
2.5. Statistical Analysis
- The statistical analysis of the qualitative and quantitative variables was either of mono-varied type, or of multivariate type using STATISTICA software. The Student test was used for the averages and the variances comparison and the Newman-Keuls test for the comparison between the rates and a significant value if p<0. 05.
3. Results and Discussion
3.1. Sample Soils Characteristics
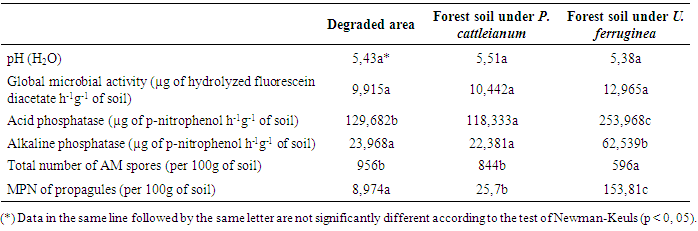
- The soil pH recorded in the invaded area is slightly higher than in the uninvaded soil but the difference is not really significant. Similarly, no relevant difference had been noticed in the global microbial activity. However, soil phosphatase activity was importantly higher on forest soil with U. ferruginea compared to those measured on the two other soils under both acid and alkaline conditions (Table 1). The high rate of acid phosphatase compared to that of alkaline phosphatase was due to the chemical characteristic of the soil (pH~5). Enzymatic activities are also disturbed by P. cattleianum. Thus, the rate of acid phosphatase measured on the invaded soil was even lower than that observed on degraded area. The density of AM spores decreased from degraded area, P. cattleianum soil, to forest soil with U. ferruginea (Table 1). Sporulation can be a sign of stressed environnements at certain point. Therefore high total number of endomycorrhizal spore in the soil can indicates that most of the endomycorrhizal fungi are in sleeping mode on invaded or degraded area. The evaluation of (MPN) for each type of soil confirms that constatation. The MPN is negatively correlated to the total number of spore. The observed rate of MPN per 100g of soil on invaded soil was six times lower than that determined on the univaded forest soil. The installation of P. cattleianum on soil induces then the inhibition of the spore germination. Our result showed the negative effect of Psidium cattleianum propagation on soil microbial activities and endomycorrhizal characteristics.
|
3.2. Plant Growth and AM Colonization
- The growth response and AM colonization of U. ferruginea seedlings were significantly stimulated on forest soil without P. cattleianum than those observed on the other area as shown in Fig. 1. These results were shown according to the analysis of the height, the root biomass and AM colonization where we noticed a high value of each of these parameters on that type of soil. However, the result showed that the development of U. ferruginea seedlings on soil forest colonized by P. cattleianum was similar to that observed on degraded soil. Although the two samples soils were taken from the same forest, the growth of U. ferruginea on soil invaded had been reduced significantly.
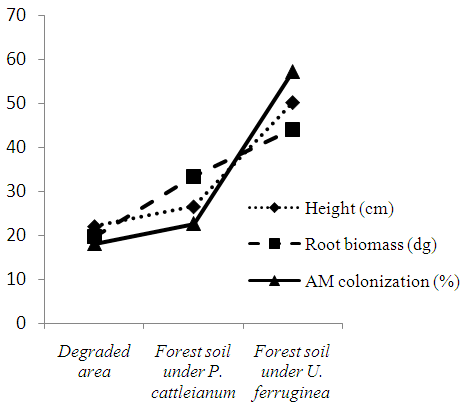 | Figure 1. Development and AM colonization of U. ferruginea seedlings on the soil types after 5 months of cultivation |
4. Conclusions
- This study provides evidence that the propagation of P. cattleianum can influence both soil microbial properties and native plant performance. The high competitive ability of this plant constitutes a real threat for biodiversity in the ecosystem. In the present study, the growth of U. ferruginea seedlings may be promoted by improving the AM colonization fungi.
AKNOWLEDGMENTS
- This work was possible with Laboratory of Environment Microbiology, CNRE Antananarivo - Madagascar and the Laboratory of Molecular Biology - University of Fianarantsoa - Madagascar.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML