-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2017; 7(1): 11-16
doi:10.5923/j.ije.20170701.02

Major Deteriorative, Pathogenic and Beneficial Fungi Reported from Various Subterranean Caves of the World: A Mini Review
Deepa Biswas1, 2, Jayant Biswas2
1Department of Life Science (Botany), Dr. C.V. Raman University, Bilaspur, C.G., India
2Central Laboratory, National Cave Research and Protection Organization, Raipur, C.G., India
Correspondence to: Jayant Biswas, Central Laboratory, National Cave Research and Protection Organization, Raipur, C.G., India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The almost unusual and sterile characteristics of the caves have always attracted the geomicrobiologits to explore noble microbes from it. Earlier the study in this field was limited, but in the recently a number of geomicrobiologists are taking interest to study various aspects of microbiology of caves. During the last few decades some incidents occurred due to the fungal outbreaks inside some caves of worldwide which create loss not only to our existing biodiversity and cultural heritage but also to human health. In the present review, we have tried to highlight some such fungi outbreak related issues which had already created a great loss to us. In addition, some beneficial aspects have also been discussed. It is the need of the time to monitor periodically the overall activities of the microbes inside every show cave so that proper strategy could be framed to save the caves whenever required.
Keywords: Fungal-outbreak, Cavernicoles,Entomopathogens, Biodeterioration, Histoplasmosis
Cite this paper: Deepa Biswas, Jayant Biswas, Major Deteriorative, Pathogenic and Beneficial Fungi Reported from Various Subterranean Caves of the World: A Mini Review, International Journal of Ecosystem, Vol. 7 No. 1, 2017, pp. 11-16. doi: 10.5923/j.ije.20170701.02.
Article Outline
1. Introduction
- Fungi are always notable for their antique variety and are known to exist in almost all sorts of environments. As per an estimation, more than 100,000 species of fungus have been described so far [1] out of which only a handful ~about 100 have been reported from caves [2]. Cave ecosystems are characterized by perpetual darkness almost constant temperature, high humidity and low accessibility of energy inputs. A complete cave system could be divided into different ecological zones based on the direct impact of their ambient external environmental conditions. Caves have mainly four ecological zones [3, 4]:(1) The twilight zone: a zone adjacent to the entrance where light intensity, humidity and temperature vary as per the external environmental conditions. (2) The transition zone: the zone remains almost dark with variable humidity and temperature. (3) The deep zone: a complete dark zone with almost 100% humidity and constant temperature. (4) The stagnant zone: a complete dark zone with 100% humidity and where there is little air exchange, and CO2 concentration may become high. Each of the above-mentioned ecological zones harbors some identical varieties of faunal species, habituated to live in that particular ecological condition. Microscopic fungi an important part of cave microflora, occurs in each ecological zone of a cave. Fungi also play a crucial role in energy input by participating in the process of decomposition of organic matter, making it available to other members of the cave community [5-7]. Nevertheless, some of the fungi are fatal for cave organisms due to their parasitic or pathogenic behaviour (explained ahead). Fungal spores which are commonly found in the caves are usually imported by the air current, water-stream and/or by some faunal movements such as bats and arthropods, or by humans visiting the same [8-12]. Mostly, fungi are restricted to only such areas which remain under the influence of its external surface. In cave till date, much stress has been given to know the ecological role of fungi only as decomposers of organic material. Nevertheless, it also serves as potential food sources for other organisms [6] which have been almost ignored. Apart from it, in the recent past year's research on cave fungi have also focused on some crucial issues related to human health [13-16].The goal of this paper is to review all the notable microscopic fungi isolated from various substrates of different cave systems and to estimate their beneficial and/or pathogenic roles with respect to the other organisms permanently or frequently uses the caves. However, in our review, we have not included such fungi which were reported either from sea or ice caves, lava tubes, tectonic rift caves, or other natural or human-made subterranean habitats.
2. Cave Fungi Injurious to the Respective Cavernicoles
- Since past few years, public awareness about the cave mycology has been found to increase manifolds with the appearance of white-nose syndrome (WNS), which was caused by the recently described fungus Geomyces destructans Gargas et al., 2009 [17]. It was first observed in the winter of 2006-2007 in New York [18, 19]. White-nose syndrome (WNS) is a disease which directly targets hibernating bats and is a white fungus which grows on the muzzle and other parts of bats, resulting in the death of the species. It has been reported that the infected bats behave strangely during cold winter months, become sick and ultimately die. This individual fungal infection has already killed more than 5.5 million bats in the Northeast America and Canada. As per a report of US Fish and wildlife services, in some areas cent percent extinction of bats has also been noted due to WNS (http://www.whitenosesyndrome.org/). Though the human beings have never been directly victimized by G.destructans, but it acts as a strong vector to spread the disease in the cave bats. Proteus anguinus, the only cave-dwelling vertebrate of Europe; under laboratory conditions, was reported to be infected by fungi from the genus Saprolegnia (ubiquitous in freshwater bodies [20]. However, whether the same history is repeating in the cave or anywhere in subterranean conduits is yet to be understood.While hibernation, cavernicolous (cave living) insects get readily infected by entomopathogenic fungi inside the caves. Such entomopathogenic fungi have drawn the attention of various researchers from a long time. A brief account of the same has already been published by Kubátová & Dvořák [21]. Further, in the same report, few entomopathogenic fungi were discovered by them in two cavernicolous moths Scoliopteryx libatrix (mostly infected by - Paecilomyces farinosus) and Triphosa dubitata (mostly infected by- Cordyceps sp.). While searching the entomopathogenic fungi carried to the cave by a cave spider, Meta ovalis, Yoder et al. [22] found two major insect pathogens; Beauveria spp. and Paecilomyces spp. However, their pathogenic status is little bit arguable, as some reports support its pathogenic nature for the spider [23] while other denied the same [22, 24]. Unfortunately, few reports strongly advocate the lethal effects of Beauveria spp. and Paecilomyces spp. to the cave cricket, Troglophilus neglectus [22, 25]. While establishing a relationship between mycoflora and Kozlov rob Cave’s crickets Troglophilus neglectus, several hyphomycetes and zygomycetes were identified from its living larvae, adults and dead bodies [25, 26]. Indeed, a new species of Mucor troglophilus was also investigated from the living adults and larvae specimen of T. neglectus [26]. In addition Zalar et al. [26] also reported 30 fungal species from a dead body of the same cricket species, but their pathogenic status has not been confirmed.
3. Cave Fungi Threats to the Human Health
- The issue of human health who visits into the caves was then highly threatened when a disease Histoplasmosis was an outbreak in the human cave-visitors of Puerto Rican caves in the mid of previous century (Year: 1960-1962). Histoplasmosis which is also known Cave disease [27], is caused due to the histoplasmosis fungus –Histoplasma capsulatum, common in soil sediments and guano depositions of bat-roosting caves. Today, over symptoms of Histoplasmosis is found among AIDS patients because of their suppressed immunity [28]. The disease primarily affects the lungs, but gradually victimized the other organs too which can be fatal if left untreated.Recently Ogorek et al. [29] reported two infectious fungi from the Harmanecka and Driny caves of Slovakia. As per the findings, Penicillium granulatum was the most dominating fungal species isolated from the guano deposition and air of both the caves, but Mucor hiemalis was found to be dominant only in the guano deposition of Driny cave. Though the authors didn’t find any risk factor among the human visitors of these caves, but reports are available which indicates that both the species are injuries for human health. As per Desai et al. [30] M.hiemalis may cause cutaneous and subcutaneous mucormycosis in humans whereas the production of secondary metabolites, including mycotoxins by P.granulatum may proof fatal to animals and humans [31]. Further, due to antigens produced from mold conidia of P.granulatum a person can be victimized of allergic alveolitis, chronic respiratory symptoms, and sensitization [32].Ogóre et al. [33] reported the Rhizopus stolonifer as the most frequently isolated fungus from the air and the rocks of the Niedźwiedzia Cave of Poland. This particular fungus is a reported cause factor for mucormycosis of lungs, sinuses and generalized mucormycosis in human [34].
4. Cave Fungi Injurious to Our Cultural Heritage
- In recent years the awareness towards the cave conservation has also been increased since the people knew that the subterranean cave serves as a storehouse of scientific facts and figures in itself. Lascaux Cave, a world heritage site for existing of valuable prehistoric paints is suffering from several microbiological crises since its discovery (1940). Within few years of its invention, it becomes a major crowd puller site of France. As a result, time to time blooming of microorganisms inside the cave became a major threatening factor for the various pigments of paintings exist inside the cave [35-38]. Besides Lascaux, today, most of the world famous rock-art caves, viz., Altamira, Tito, Bustiro, Ajant and Ellora are suffering from biodeterioration. To proper understand the complex processes of microbial colonization and biodeterioration of the cave and its paintings various investigations have been carried out [7, 37, 39-48].
5. Notable Beneficial Fungi from the Worldwide Caves
- The inherent properties of the caves, i.e., the constant geophysical characteristics and high relative humidity, are some of the major factors that have been encashed by various countries in the nineteenth century for cultivating edible fungi [2]. Fungi are the major saprotrophs in the cave communities plays a major role in its food web by contributing in the decomposition of organic matter and providing it to the various members of the cave community. Fungal spores and mycelia are the constitutions of diet for various cavernicolous insects [49-51]. Various works [52, 53] have also recognized the fungi as an essential food source for many of cave arthropods communities.
6. Conclusions
- Most of the cave fungi discovered yet are as saprobic, which usually depends on the remains of dead organisms or other organic sources available in such food-starved environment of caves. Unfortunately, few of them also proliferate as parasites, relying on living organisms for food [1]. These are key factors which cause the deterioration of the ambient environment. Indeed, inside the cave, the only limiting factors for the proliferation of fungi are the lack of light which check the autotrophs to grow. The commonly found fungi from the caves are the fungal genera, such as Fusarium, Neonectria, Penicillium, Aspergillus, Cladosporium etc. which are indeed the soil saprophytes [54-56]. However among them, only sometimes few fungi substantiate themselves either harmful for the human health, pathogenic for the existing cavernicoles or deteriorate for the complete cave ecosystem. Though various types of cultural and/or scientific heritages are the part of cave ecosystems, thus in that respect those heritages also are threatened by the same issue.However, the caves should not be much threatened from the externally imported spores (fungi), because various studies have already reported that inside the cave, the dispersion patterns of the spores of any microorganism is tightly associated with the distance of its nearest entrance [57, 58]. In other words, the limitation for the occurrences of any deteriorative fungi would be only to that extent, up till where the air flow exists or any other physical factor(s) which could promote the spore dispersion inside it.
7. Future Directions
- During the visit of any tourist/virgin cave, we came across several new structures formed either by mycelia or the other parts of fungi which seem interesting for us (Figure -1). Perhaps, such types of structures we generally failed to notice in open external conditions. Indeed such structures mostly occur in the initial zones of the caves as due to less circulation of air inside the cave the dispersal of the spores could not become possible for the deeper parts of the zones. We must carefully collect such samples and submit to some microbiological laboratory for proper identifications, so that a database regarding the existing fungi is maintained and according to which cave conservation strategies could be prepared whenever to feel necessary.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML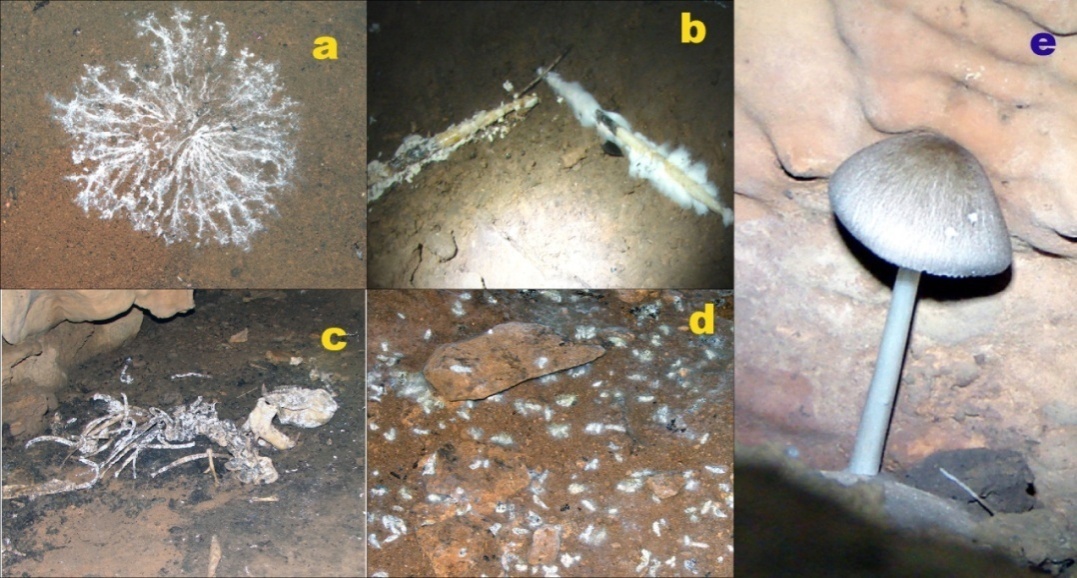
 43(3), 295-303.
43(3), 295-303.