-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2016; 6(3): 47-54
doi:10.5923/j.ije.20160603.01

Ecological Assessment of Brewery Effluent Impact on the Macrobenthic Invertebrates of Ikpoba River, Edo State, Nigeria
Albert C. Ibezute1, Godwin I. Asibor1, Sandra U. Ibezute2
1Department of Environmental Management and Toxicology, Federal University of Petroleum Resources, Effurun, Nigeria
2Department of Animal and Environmental and Biology, University of Benin, Benin City, Nigeria
Correspondence to: Albert C. Ibezute, Department of Environmental Management and Toxicology, Federal University of Petroleum Resources, Effurun, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

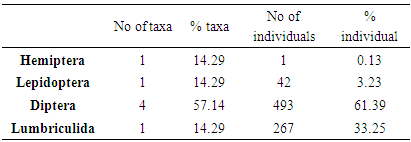
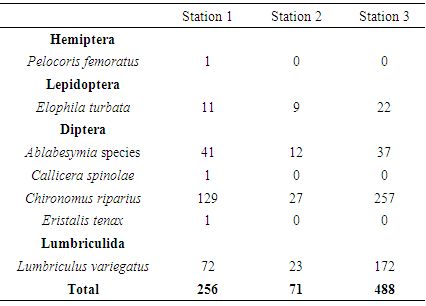
An investigation into the impact of brewery factory effluent to the macrobenthic assemblage in Ikpoba River was carried out from the months of April to September 2012. Samples were collected from different three sites, designated as station 1 (upstream of effluent discharge point), station 2 (effluent discharge point) and station 3 (downstream of effluent discharge point). The collected samples were analyzed for the physicochemical characteristics while bank root substrate samples were sorted, identified and counted for macrobenthic composition. A total of seven macrobenthic invertebrate species comprised of 803 individuals were identified. These include four species of Diptera and one species each for Hemiptera, Lepidoptera, and Lepidoptera. Among the macrobenthos, Diptera dominated contributing 61.39% of the total density occurrence, while Lumbriculida, Lepidoptera and Hemiptera, made up of 33.25%, 3.23% and 0.13, respectively. The highest total number of taxa was observed in station 1 with a total of seven taxa with 256 individuals; whereas, station 2 and station 3 each with a total of four taxa with 71 and 488 individuals, respectively. The changes observed in some of the macrobenthic assemblages of Ikpoba River, notably increased indicating inefficient effluent treatment in the breweries. This study has also shown that the quality of receiving water is influenced significantly by the chemical composition of effluents discharged into the River.
Keywords: Brewery factory effluent, Macrobenthic Invertebrates, Ikpoba River, Water quality, Aquatic pollution
Cite this paper: Albert C. Ibezute, Godwin I. Asibor, Sandra U. Ibezute, Ecological Assessment of Brewery Effluent Impact on the Macrobenthic Invertebrates of Ikpoba River, Edo State, Nigeria, International Journal of Ecosystem, Vol. 6 No. 3, 2016, pp. 47-54. doi: 10.5923/j.ije.20160603.01.
Article Outline
1. Introduction
- In the past two decades, there has been a tremendous fear of possible global biodiversity crisis arising out of a rapidly accelerating loss of species, populations and natural habitats such as tropical rainforests and wetlands. It has been estimated that more than half of the habitable surface of the planet has already been altered by human activity [1]. McCarthy [2] also suggested that we are on the verge of mass extinctions of species. These concerns stem from the realization that our knowledge of the diversity and variability of plants, animals, microorganisms and the ecosystems in which they occur is woefully incomplete.All water bodies, either lentic or lotic, contain vast numbers of invertebrate macrofauna (species visible to the unaided eye) which are used to assess water quality in rivers [3]. Not only are they numerically abundant, but they are also taxonomically diverse. Hynes [4] observed that the aquatic animals, which live on, in or near the substratum of running waters, include the nematodes, annelids, insects, crustaceans and molluscs. These organisms are important in the aquatic ecosystem because they form part of the aquatic food chain. They are an essential element of the food chain, especially for larger aquatic animals. They feed on algae and bacteria which occupy the bottom of the food chain. Biological communities have been seen as effective tools for assessing organic pollution. Macrobenthic animals can be sampled quantitatively and are easy to monitor because they respond to man-made disturbance. However, the assessment of biological communities present in an aquatic environment also reflects the quality of the ecosystem [5]. The monitoring of biological communities can be done at a variety of trophic levels including micro-organisms (bacteria, protists, and viruses), primary producers (algae and vascular plants), primary consumers (invertebrates) and secondary consumers (fish) [6, 7]. Macrobenthic Invertebrate assemblages are useful indicators of localized conditions because many have limited migration patterns or are sessile (non-motile). Thus, are helpful in examining site-specific impacts [5]. Changes in the environment will be reflected in variations in the species assemblage both spatially and temporally (i.e., affected and unaffected sites over time). Some studies on macrobenthic invertebrates and other related topics have been carried out in zone Nigerian rivers [8, 9, 10]. Although studies on macrobenthic invertebrates communities in rivers in response to brewery effluent have not been given much attention in Nigeria and Africa in general, Ogbeibu and Egborge [8] recorded a total of 214 invertebrate taxa in the Okomu Reserve, comprising 80 zooplankton and 134 macrobenthic invertebrates. Evagelopoulos et al. [11] studied the spatial variations of both phytoplankton and macrobenthic invertebrates descriptors (composition, abundance, and biomass) at six sampling sites in the low-salinity ponds of Kalloni Saltworks. Their studies revealed 54 macrobenthic invertebrate taxa belonging to 5 groups. Pearson and Rosenberg [12] noted that organic enrichment of sediments as a r esult of sewage and other organic contaminants may lead to a series of non-linear changes in the abundance, biomass and diversity of macrobenthic invertebrate, in both spatial and temporal patterns. Although the composition, distribution and abundance of macrobenthic invertebrates in Nigeria have been reported in some studies [13, 14, 15]. However, no study has been carried out to assess the impact of brewery factory effluent on the macrobenthic invertebrate community of Ikpoba River, hence, the need for this study. This study is therefore aimed at evaluating the impact of brewery effluent on the macrobenthic invertebrate of Ikpoba River, and to establish a relationship between the current physicochemical properties and the macrobenthic invertebrate of Ikpoba River.
2. Materials and Methods
2.1. Study Area
- Ikpoba River, a fourth order stream, is located in Benin City, Edo State in South Nigeria (Lat 6.5ºN, Long 5.8ºE). Its headwater originates from North West of Benin City and flows north to south through the city (Benka-Coker and Ojior, 1995). The river flows through a dense rain forest where the allochthonous input of organic matter from the surrounding vegetation is derived through run-off from the surface of the soil. Typically, the region have the characteristic features of the humid tropical wet and dry climate governed primarily by the rainfall. The vegetation of Ikpoba River consists of rainforest which is secondary in nature and has been greatly subjected to deforestation and other anthropogenic activities. The main vegetation consists of rubber palm, bamboo plant; and palm trees with their symbiont epiphytic ferns.
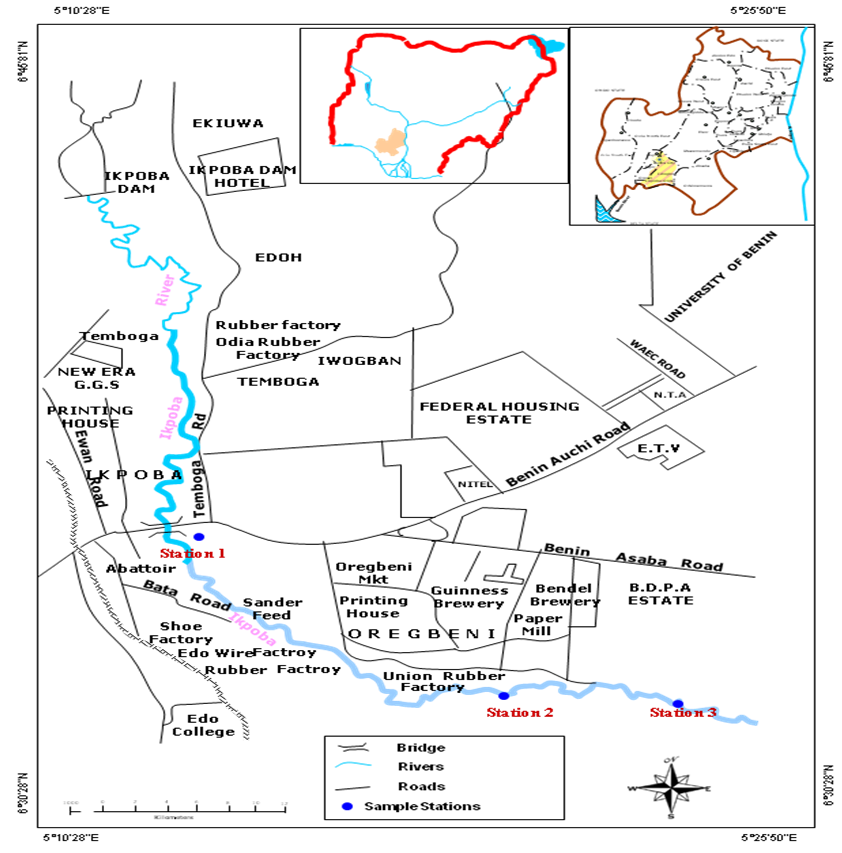 | Figure 1. Map of the study area (Map of Nigeria and Edo State as inset) |
2.2. Sampling Stations
- Three sampling stations were selected for this study. Station 1 is the upstream section of the river, the point, just after the bridge. Water velocity is high, and depth is minimal when compared to other stations. At this section the water is clear, but the substratum is silted. Human activities include fishing and domestic activities such as bathing and washing of clothes and cars, mechanic workshops and the activities of the local deity shrine. Station 2, was located 400m downstream of station 1. It was the contact point of the brewery effluent with the river water before it flows down to station 3. The substratum is coarsely sandy with pebbles. The river bank is muddy and has a large deposit of spent grain which has accumulated over time as a result of the effluent flow. The water is highly turbid, and distinct odour of decaying organic materials. The activities in this region include fishing. Station 3 was located about 100m downstream of station 2. The water is also turbid similar to that of station 2. The activities in this station include distillation of palm wine to alcoholic beverages, fishing and farming activities.
2.3. Collection of Water and Bankroot Samples
- Monthly sampling of Ikpoba River was undertaken throughout the wet season months from April to September 2012. Samples were collected between the hours of 0900 and 1200 on each sampling day and at each time, sample collection commenced in station 1 and terminated in station 3. Standard methods and instrumentations were followed during sample collection procedures [17]. At each station, the surface water temperature was taken in-situ. Surface water samples for physicochemical analyses were collected into thoroughly cleaned 1liter polyethylene bottles and tightly closed. Each bottle was rinsed with the appropriate sample before the final sample collection. The samples were placed in a cooler box and then taken to the laboratory for analyses. For dissolved oxygen (DO) determinations, separate samples were collected in 300 ml plain glass bottles and the samples fixed using the azide modification of Winkler’s method [17]. Samples for biochemical oxygen demand (BOD) were collected into dark glass bottles for incubation and subsequent DO determination. In the laboratory, pH, total dissolved solids, conductivity, turbidity, dissolved oxygen, biochemical oxygen demand (BOD5), chemical oxygen demand (COD), chloride, sodium, potassium, magnesium, sulphate, nitrate, phosphate, lead, iron, zinc, cadmium and copper were determined according to procedures outlined in the Standard Methods for the Examination of Water and Wastewater [17]. The pH was measured using HACH digital meter, total dissolved solid was determined using a TDS meter (Model 4076), conductivity was measured using Cybersan 510 conductivity meter, while turbidity was determined using a DR/2000 HACH spectrophotometer. The titrimetric method was applied in the determination of chemical oxygen demand (COD), chloride, sodium, potassium, and magnesium. Sulphate, nitrate, and phosphate were determined spectrophotometrically at 380nm, 470nm, and 680nm respectively. Heavy metal such as lead, iron, zinc, cadmium and copper in the water sample was determined using Atomic Absorption Spectrophotometer (Buck Scientific Model-210) at 217.0nm, 248.0nm, 213.9nm, 228.8nm and 324.8nm respectively.The modified ‘Kick sampling technique’ [18, 19] was used in collecting benthic macroinvertebrates from the bank root biotope of each station. A hand net (154mm mesh size) was used in sampling 0.25m2 of the substratum at four different points to the form of one composite sample per station. Collected samples were preserved with 10% formaldehyde in polyethylene containers. In the laboratory, macrobenthic invertebrates in the sample from the three stations were sorted out with the aid of a binocular dissecting microscope (25-40X American Optical Binocular microscope, model 570) and preserved in 4% formalin. The observed specimens were identified to the species level with the aid of identification keys [20, 21] while counting was done manually.
2.4. Statistical Analysis
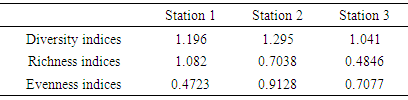
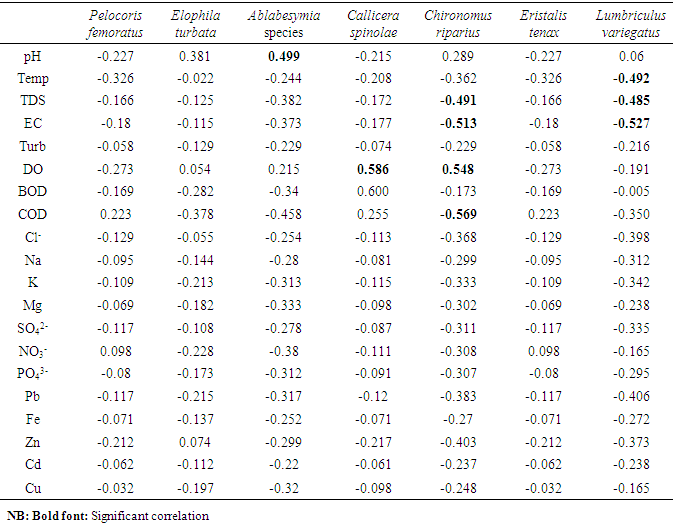
- Statistical package for Social Sciences (SPSS 16.0) to test for significant differences in the physicochemical parameters using One-way analysis of variance (ANOVA), where significant values (p < 0.05) were obtained, ‘A posteriori’ Duncan Multiple Range Test was subsequently applied to all pairs of means to detect the location of difference. Palaeontological statistical software package for education and data analysis (PAST 1.97) was used to evaluate relationships (such as diversity indices, taxa richness and species evenness) between macrobenthic invertebrates, while pearson correlation was used to establish comparison between macrobenthic assemblage and physicaochemical parameters.
3. Results and Discussion
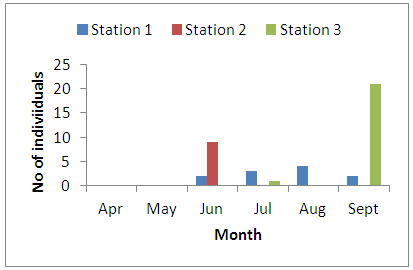
- Physico-chemical parameters (water temperature, total dissolved solids, electric conductivity, turbidity, BOD, COD, pH and DO) and chemical parameters (chloride, sodium, potassium, magnesium, sulphate, nitrate, phosphate, lead, iron, zinc, and cadmium) were found highest (p<0.05) at the discharge point (Station 2) than the upstream (Stations 1) and downstream (Station 3) stations. This result confirms previous reports on some aquatic studies [25]. The low dissolved oxygen concentration recorded agreed with values reported for some Nigerian waters [26]. The low dissolved oxygen values revealed anoxic condition during the study period. Such low oxygen concentration has been reported in Eruvbie Stream and Ikpoba River as a result of the discharge of organic-rich effluent [25, 27]. Low dissolved oxygen has been reported to be deleterious to most aquatic fauna [33]. Hemiptera represented by Pelocoris femoratus was present only at station 2 in the month of May (Fig 2). Lepidoptera represented only by Elophila turbata was present in all stations. It appeared in station 1 throughout the months of June, July, August, and September with the highest value reported in August (Fig 3). It appeared only once in station 2 at the month of June; in station 3, Elophila turbata appeared briefly in low quantity in July while a higher number was reported in September. Diptera was represented by Ablabesymia species, Callicera spinolae, Chironomus riparius and Eristalis tenax. These macrobenthos species in station 1 increased steadily throughout the period of study. In station 2, dipterans appeared in the months of July until August. Whereas, in station 3, it was present in higher number than the other stations from the month of June to September. Lumbriculida was represented only by Lumbriculus variegatus. It was present in station 3 throughout the period of study. It decreased in number from April to September (Fig 5).
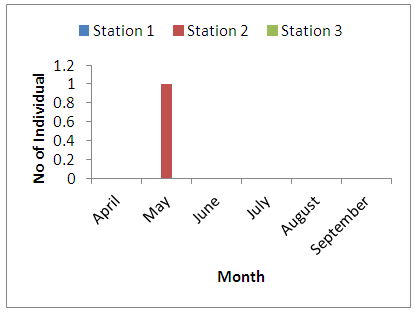 | Figure 2. Monthly variation of the family Hemiptera |
 | Figure 3. Monthly variation of the family Lepidoptera |
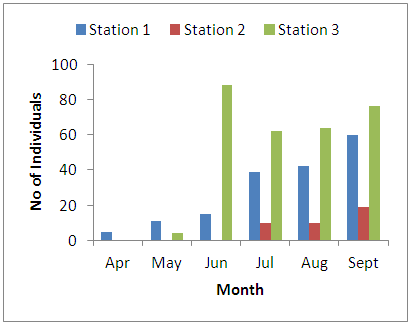 | Figure 4. Monthly variation of the family Diptera |
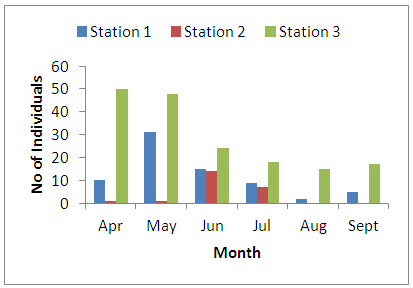 | Figure 5. Monthly variation of the family Lumbroculida |
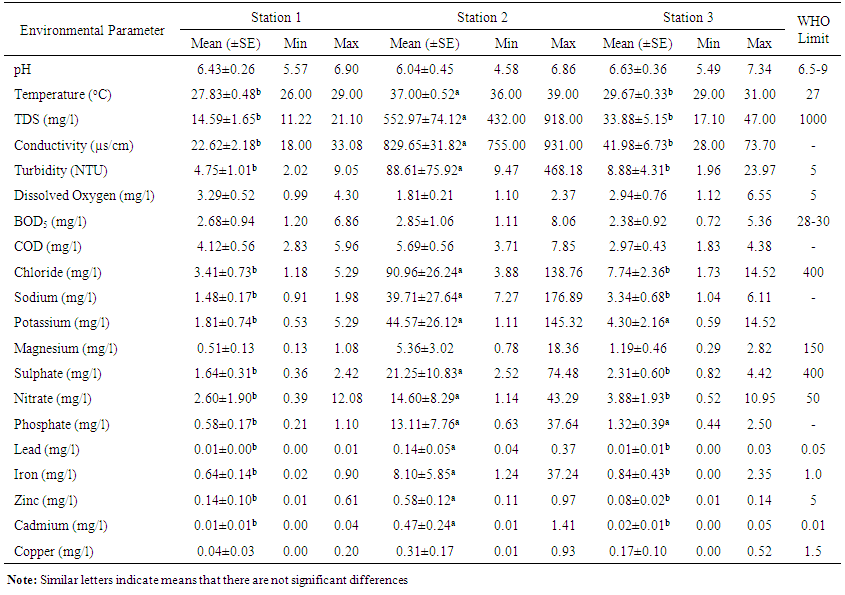 | Table 1. Some physical and chemical conditions of Ikpoba River water at three sampling stations from April to September 2012 |
|
|
|
|
4. Conclusions
- The changes observed in some of the macrobenthic assemblages of Ikpoba River, notably increased indicating inefficient effluent treatment in the breweries. This study has also shown that the quality of receiving water is influenced significantly by the chemical composition of effluents discharged into the River. High values of diversity indexes in the upstream sections of Ikpoba River reflected the stability of the physical and chemical characteristics of the river in the upstream sections. On the other hand, low values of diversity found in the downstream sections may be due to the increased influence of human activities. Because Ikpoba River serves as a source of domestic water supply for the local community for drinking, washing, fishing and swimming, all specified quality criteria must be met by the breweries especially before the discharge.
ACKNOWLEDGEMENTS
- We are grateful to Professor CG Orosaye of the Department of Animal and Environmental Biology, the University of Benin for guiding us with this research; and also Mr. Bayo Lawal of Tudaka Environmental consultancy Laboratory for assisting us with the laboratory analysis during this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML