-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2014; 4(4): 176-183
doi:10.5923/j.ije.20140404.03
Aflatoxicosis in Broilers: Efficacy of a Commercial Mycotoxin Binder on Performance and Immunity Parameters
Hedayati M., M. Manafi, M. Yari
Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran
Correspondence to: Hedayati M., Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
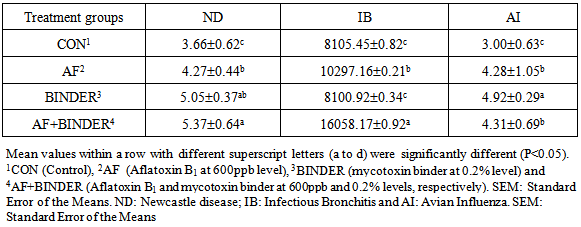
Experimental mycotoxicosis was induced into broiler chickens by feeding 0.6ppm aflatoxin B1 (AFB1) and 0.2% commercial mycotoxin binder (BINDER) from 0 to 42 days of age using 240 day old chicks to evaluate the body weight, feed consumption, FCR and antibody response against ND, IB and AI. AF fed birds showed a clear indication of aflatoxicosis by reduces in body weight, feed consumption and increase in FCR almost at all weeks. The antibody titers are also affected by incorporation of AF into the diet at 42 days of age. The addition of binder could significantly alter the adverse effects of AF and in absence of AF, when binder alone was fed to chicks; the better performance was recorded, when compared with control group. The study revealed that AF and binder in combination could act cumulatively and adversely affect the health of broiler chicken.
Keywords: Aflatoxin B1, Mycotoxin binder, Body weight, Feed consumption, FCR, Antibody titers, Broilers
Cite this paper: Hedayati M., M. Manafi, M. Yari, Aflatoxicosis in Broilers: Efficacy of a Commercial Mycotoxin Binder on Performance and Immunity Parameters, International Journal of Ecosystem, Vol. 4 No. 4, 2014, pp. 176-183. doi: 10.5923/j.ije.20140404.03.
Article Outline
1. Introduction
- The poultry industry has gained much interest as an important economic activity across the globe. Mycotoxins are the toxic metabolites produced by certain fungi. They are always a hazard to man and domestic animals and had come to public interest since the past 30 years. Among them, Aflatoxins are the most dangerous toxin produced by Aspergillus flavus and Aspergillus parasiticus, species of fungi on foods and feeds. The disease which aflatoxin causes is called aflatoxicosis. The factors influencing occurrence of aflatoxins are certain environmental conditions; hence the extent of contamination will vary with geographic location, farming methods and the susceptibility of commodities to fungal invasion during pre-harvest, storage, and processing periods (Wan et al., 2013; Manafi and Khosravinia, 2013). Many countries have attempted to limit exposure to aflatoxins by imposing regulatory limits on commodities intended for use as food and feed. The mycotoxins are known to have strong hepatotoxic and carcinogenic effects and are regulated by feed/food law in at least 100 countries. Numerous reports on effects of aflatoxins on bird performance and serum chemistry have been previously reviewed by many scientists (fig. 1). There is a general agreement that dietary aflatoxins reduce weight gain, feed intake, and increase feed conversion ratio. A study by Dersjant-Li et al. (2003) reported that each ppm of AFB1 in diet would decrease the growth performance of broilers by 5%. However, the data presented in last decade is not consistent with this general term. For instance, Raju and Devegowda (2002) reported a 21% decrease in body weight of broilers fed 300ppb AFB1 in their diet. Contrary to this, Tedesco et al. (2004) noted a reduction at the rate of only 10% in weight gain of broilers at 28 days fed 0.8ppm AFB1. In higher levels of 3ppm AFB1, only 11% reduction in final body weight was reported by Valdivia et al. (2001). From all these reports, it is obvious that both the level and length of AFB1 exposure affect the amount of reduction in weight gain of broilers. Next to this, effects on liver, the immunosuppressive nature of AFB1 is the best recognized area of its toxicity. Recent data indicates high correlation between outbreaks of Newcastle disease (ND) and aflatoxin contamination in broilers (Yunus et al., 2008). Commonly, the immune-toxic dose of AFB1 is considered as less than the dose required provoking a reduction in bird performance. The edge dose of AFB1 may be generalized to be 0.4 and 1mg/kg for the negative effects on cell mediated and humoral immunity, respectively (Galvano et al., 2005).
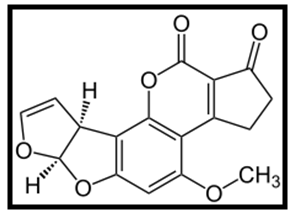 | Figure 1. Chemical structure of aflatoxin B1 |
2. Materials and Methods
- This experiment was planned and carried out in the Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran with objective of evaluating the performance and immune response of broilers fed with aflatoxin B1 and a mycotoxin binder.
2.1. Experimental Design, Housing, Management and Test Diet
- 240 day-old unsexed Ross 308 strain of broiler chicks were wing banded, weighed and randomly spread in a completely randomized experimental design with four treatments and three replications of twenty chicks in each. Each replicate group of chicks was housed in an independent pen, conventional deep litter house. Chicks in all the replicates were kept up to six weeks of age under uniform standard conditions. Brooding was done till three weeks of age. Each pen was fitted with an automatic bell type drinker and a hanging tubular feeder. Chicks were provided ad libitum feed and water throughout the study. Feeding of test diets commenced at first day of age and continued till the termination of experiment at six weeks of age. The temperature was maintained at 30±1°C in the first week and reduced by 2.5°C per week to 21°C. From day one until day 4, the lighting schedule was 24 hour. At days 14-42 the dark time was gradually increased to 4 hour. Diets were prepared to meet the nutrient requirements of commercial broilers during the starter (0-2 weeks), grower (2-4 weeks) and finisher (4-6 weeks) periods. The composition of diets was adopted from NRC, (1994) and is presented in Table 1. The basal diet was formulated using commonly available feed ingredients which were screened for AF prior to the formulation of diets and it is found to be around 280ppb in normal feed. The Aflatoxin B1 was procured from Sigma Aldrich, USA and diluted to reach to the required level of administration. The experimental diets were prepared by adding required quantity of aflatoxin to arrive at the level of 600ppb of AFB1. Diets were prepared without addition of aflatoxin and binder as Control (group 1); 600 ppb Aflatoxin B1 (group 2); 0.2% of binder (group 3) and 600ppb Aflatoxin B1 + 0.2% of binder (group 4). Niltox, the mycotoxin binder used in this study is a unique composition of minerals (extra purified clay containing diatomaceous earth mineral), antioxidants (Curcuminoids extracted from Turmeric) and enzymes (Epoxidase and Esterase), a property product of Zeus Biotech Limited, Mysore, India. It is claimed that incorporation of this product in poultry diets would effectively prevent DNA adduct formation and cellular damages in the biological systems through degrading peroxides, amides and lacto rings in non-polar toxins such as aflatoxins. This study was undertaken to evaluate the efficacy of a mycotoxin binder for counteracting AFB1 in experimentally contaminated broiler breeder diets.
|
2.2. Vaccination Schedule
- The local office of Iranian Veterinary Organization has proposed the required vaccination which is modulated by the veterinarian of Department of Animal Science, Malayer University, as below:vaccination for Newcastle Disease (ND) virus happened three times: first spray at one days old of chicken in breeder farm, second on the 13th day as B1, BRONHOPEST B1 SPF (VETERINA®, Zagreb, Croatia) and (CEVA®, Libourne, France) in drinking water and their booster on 20th day as clone-30 (HIPRAVIAR® CLON, Amer, Spain) through drinking water. Vaccination against Bronchitis virus happened in two times as the following: first spray at commencement of the experiment and it’s booster in drinking water on the 10th day, both as H-120 (CEVA®, Libourne, France). Vaccination against Infectious Bronchitis (IB) virus happened in two times: first on day 16 and the second on the 23th day, both as Gambo-l (CEVA®, Libourne, France) in drinking water. The sera were applied to HI test in 28 days, to determine Ab to NDV. In titers lower than 5, the booster B1, BRONHOPEST B1 SPF (VETERINA®, Zagreb, Croatia) was administrated in drinking water for broilers.
2.3. Studied Parameters
- Performance parametersBody weight and cumulative feed consumption were recorded and feed conversion ratio (FCR) were calculated week wise. All chickens were weighed individually at the end of each week till week VI, by digital electronic top pan balance with 0.01g accuracy to record body weight. Feed consumption was recorded replicate-wise each week in all pens till 6 weeks of age and feed consumption per bird was calculated. Weekly FCR was calculated up to 6 weeks, as feed consumed per unit body weight gain.
2.4. Immunity Parameters
- At the end of the trials, upon obtaining the permission of Ethical Committee of the University, six birds from each replicate were sacrificed by cutting the jugular vein and blood samples were individually collected in 10-mL heparinized tubes and stored on ice for hematology analysis. Blood was centrifuged @4000 rpm for 10 min and serum separated after 8 to 10 hours as per the standard procedures (Calnek et al., 1992) and was stored at –20 ºC for subsequent analysis. The individual serum samples were analyzed for antibody titers against Newcastle disease (ND), Infectious Bronchitis (IB) and Avian Influenza (AI) by ELISA technique (bio check®) using an automatic analyzer (Boehringer Mannhein Hitachi 704 automatic analyzer, Japan). Treatment-wise means of titers were computed.
2.5. Statistical Analysis
- The total experimental data were statistically analyzed using the General Linear Model procedure of the Statistical Analysis System (SAS®) software (SAS Institute, USA, 2000). Overall data were analyzed using one way ANOVA test. Duncan multiple comparisons range test with 0.05 significance level was employed for comparison of the means (Duncan, 1955).
3. Results and Discussion
- The effects of aflatoxin and mycotoxin binder on broilers have been shown in Table 2. It is found that, at the time of initiation of trial, all chicks were in almost similar range and uniform. At the end of first week, there was a significant reduction in body weight of broilers fed AF. In group fed binder, the body weight has shown no significant changes, when compared with control group. In AF+BINDER group, adverse effects of aflatoxin which was seen in AF group, could not significantly (P<0.05) alleviated. At day 14, the AF fed group had significantly (P<0.05) lower body weight, compared with control group. The binder fed group has shown the best body weight among all treatments and addition of binder to AF could significantly (P<0.05) increase the body weight of broilers. AT the end of 21days, the AF fed group brought down the body weight significantly (P<0.05) up to 69 grams and addition of binder could increase the body weight for 22 grams. The maximum BW seen in group fed binder alone. At day 28 of trial, the gap between the AF and control group has been increased and addition of binder could significantly (P<0.05) restore the BW of broilers. At the end of week 6, the final BW of broilers fed AF was found to be 2052g and compared with that of control group (2207g), there was a significant decrease in AF fed groups. Addition of toxin binder to AF contaminated diet had significantly (P<0.05) increased the BW of broilers at 42 days and reached much closed to control group. The binder alone fed group has shown the highest BW (2275g) which stands for the best BW among all treatment groups.
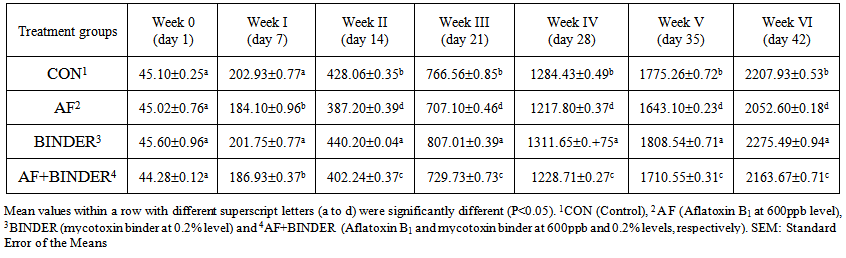 | Table 2. Body weight (g) of chicks fed Aflatoxin B1 and Mycotoxin Binder (Mean±SE) |
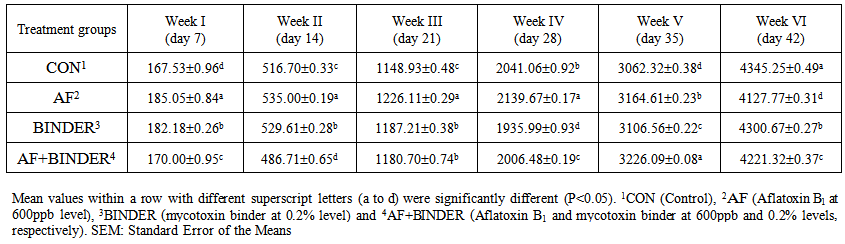 | Table 3. Cumulative Feed Consumption (g/bird) of chicks fed Aflatoxin B1 and Mycotoxin Binder (Mean±SE) |
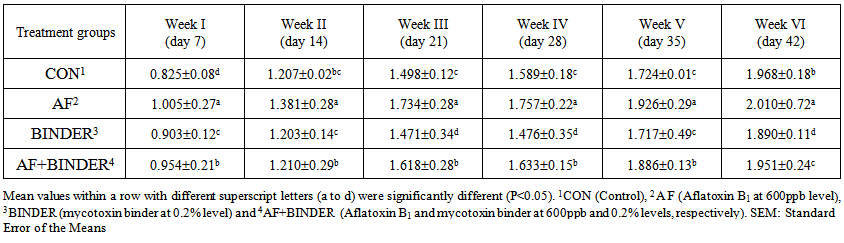 | Table 4. Feed conversion ratio (FCR) of chicks fed Aflatoxin B1 and Mycotoxin Binder (Mean±SE) |
|
4. Conclusions
- It could be concluded that aflatoxin B1 in the broiler diet can influence the performance and immune response and addition of a mycotoxin binder containing curcumin, enzymes and minerals could significantly reestablish these harmful effects on broilers. Nevertheless, there is lack in the proof of its beneficial impacts in nutrient digestibility and gut function of broilers.
ACKNOWLEDGEMENTS
- This study was funded by Directorate of Research, Malayer University, Malayer, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML