-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2014; 4(3): 135-149
doi:10.5923/j.ije.20140403.06
Small Mammal Use of Refugia, Population Recovery, and Survival Following Prescribed Burning in Scrubby Flatwoods Ecosystem, Florida, USA
Jose L Silva-Lugo
Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida 32611, USA
Correspondence to: Jose L Silva-Lugo, Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida 32611, USA.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study was conducted in Cedar Key Scrub State Reserve, Florida, USA, with the purpose of establishing: (1) if small mammals used wetlands as refugia following prescribed fire, (2) if small mammals returned to burned areas after the regrowth of the vegetation, and (3) if prescribed burning had a negative effect on the survival of the species. Few studies have addressed these topics in the literature, which are important for management and restoration purposes. The design consisted of two treatments and two control sites (scrub) with 100 traps each and a wetland next to each site with two transects (10 traps each) between the scrub and the wetland. A total of 182 individuals of Florida mouse(Podomys floridanus), cotton rat(Sigmodon hispidus), cotton mice (Peromyscus gossypinus), and golden mouse (Ochrotomys nuttalli) were marked to monitor movements between the scrub and the wetlands, but only Florida mouse and cotton rat had sufficient data for analysis. The survival analysis was carried out by using Cormack-Jolly-Seber model and the program MARK. In treatment sites, Florida mouse and cotton rat were captured primarily in the scrub (69%) before burning, they used the vegetation surrounding wetlands as refugia for 11 months after burning, and they returned to the scrub after that. In control sites, Florida mouse and cotton rat were captured mostly in the scrub (89%). Both species returned to the scrub after the vegetation had >50% cover and provided flowers and fruits. The survival analysis found that fire did not have a negative effect on the survival of the species. The results imply that if no refugia are provided during prescribed fire, wetlands next to the burned areas should not be burned. Wetlands should be burned if other refugia are provided or a year after mice have returned to burned areas.
Keywords: Prescribed burning effects, Scrub, Refugia, Survival, Small mammals
Cite this paper: Jose L Silva-Lugo, Small Mammal Use of Refugia, Population Recovery, and Survival Following Prescribed Burning in Scrubby Flatwoods Ecosystem, Florida, USA, International Journal of Ecosystem, Vol. 4 No. 3, 2014, pp. 135-149. doi: 10.5923/j.ije.20140403.06.
Article Outline
1. Introduction
- Prescribed fire is the primary method of fuel reduction in the United States, and the effects of prescribed burning on fauna need more research for this reason. Of five group of vertebrates (fishes, amphibians, reptiles, birds, and mammals), small mammals have received more attention. Ream [1] and Smith [2] reviewed 674 papers and Smith summarized the general effects of prescribed fire on small mammals. Most of these papers evaluated change in abundance/densities and dispersal to unburned/burned areas as a consequence of vegetation alteration after fire. Few studies were regarded the use of surrounding unburned habitats as refugia, or the influence of the regrowth of the vegetation on recolonization, and no study pertained to survival analysis. These three aspects are relevant because of their direct implications to wildlife management and ecosystem restoration.Studies on the use of adjacent unburned habitats as refugia after prescribed fire are rare. Goatcher [3] and Blanchard [4] carried out research of the possible use of stream-terrace hardwood forest as refuge for the cotton mouse (Peromyscus gossypinus) in Lee Memorial Forest, Baton Rouge, Louisiana. Movements across the fire-break were not detected and the investigators concluded that cotton mice did not use stream-terrace hardwood forest as refuge after prescribed fire. In contrast, other studies reported small mammal use of refugia during and after a patchy burning. McGee [5] reported that unburned and partially burned sites served as refugia to 11 species of small mammals in sagebrush communities in Burro Hill, Bridger-Teton National Park, Wyoming. Schwilk and Keely [6] found that a wildfire in California chaparral and coastal sage scrub left patches of vegetation lightly burned, which acted as refugia and enable eight species of small mammals to colonize burned sites during the first six months after fire. More research on this topic is needed because several studies have documented dispersal to unburned areas [7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. If so, most likely small mammals used other unburned habitats as temporary refugia. In Florida, only 11 studies have evaluated the effects of fire on small mammals, and no study assessed the importance of adjacent habitats to burned sites as refugia. Studies about small mammals recolonizing burned sites after the regrowth of the vegetation several months or years after prescribed burning are also rare. The reappearance of eastern harvest mouse (Reithrodontomys humulis) and cotton rat (Sigmodon hispidus) on the burned area of slash/longleaf pine habitat in north-central Florida appeared to be correlated with redevelopment of the ground cover [17]. Deer mouse (Peromyscus maniculatus) and Uinta ground squirrel (Spermophilus armatus) populations approached control numbers after 3 years when total cover of the understory was near control levels in Burro Hill, Bridger-Teton National Park, Wyoming [5]. In central Appalachians, Pennsylvania, the differences in small mammal abundance between unburned and burned sites disappeared within eight months after fire due to the fast regrowth of ground cover [18]. Ahlgren [19] in Minnesota and Sullivan and Boateng [20] in British Columbia found a decrease in southern red-backed vole (Clethrionomys gapperi) numbers 2-3 years following fire until recovery of the ground cover vegetation occurred. Research on recovery of small mammal populations relative to recovery rate of vegetation structure following fire is highly needed [21]. Few studies [22, 23, 24, 25, 26, 27, 28] have provided information about survival rate and recapture probabilities of small mammals before and after prescribed burning. This approach might be critical for population survival and for a better understanding of factors that affect population dynamics of small mammals. The objectives of this study were to evaluate the importance of vegetation surrounding wetlands next to burned sites as refugia, to determine whether population recovery is linked with the regrowth of the vegetation, and to estimate survival probabilities before and after prescribed burning. These topics are important and can contribute to a broader understanding of prescribed burning effects and their influence on ecosystem restoration.
2. Materials and Methods
2.1. Study Area and Species
- Scrub is a distinctive and one of the most threatened ecosystems in Florida [29, 30]. It is distinct because it supports a high number of threatened and endangered plants and animals [20, 29, 31, 32]. It is threatened because of natural fragmentation, human perturbations, and fire exclusion [29]. Scrub is a pyrogenic ecosystem that requires catastrophic fire for self-maintenance. Scrub fires are devastating, resulting in extensive consumption of the above ground vegetation. The natural frequency of fires is one every 10-100 years [29] or one every 20-50 years [33]. However, natural fire no longer occurs with the same intensity and frequency because the scrub has been fragmented and reduced. Conservation of this unique ecosystem relies on management and research for conservation and restoration purposes. Particularly, research on animal responses to prescribed fire is strongly needed in several public lands in Florida. One of these lands is Cedar Key Scrub State Reserve (CKSSR), in which this study was conducted. Although prescribed burning has been an important management tool for ecosystem restoration in CKSSR, this study was the first research that analyzed the effect of prescribed burning on small mammals. CKSSR (29°11’21”N 83°2’4”W; Figure 1) is located in Levy County, Florida, which has an area of 1973 ha. Cedar Key has a warm and humid climate, and based on 30 years of weather records (Weather.com), annual temperature and precipitation average 20.8°C and 126.3 mm, respectively. The heaviest rainfall takes place from June to September with some precipitation in all months of the year.
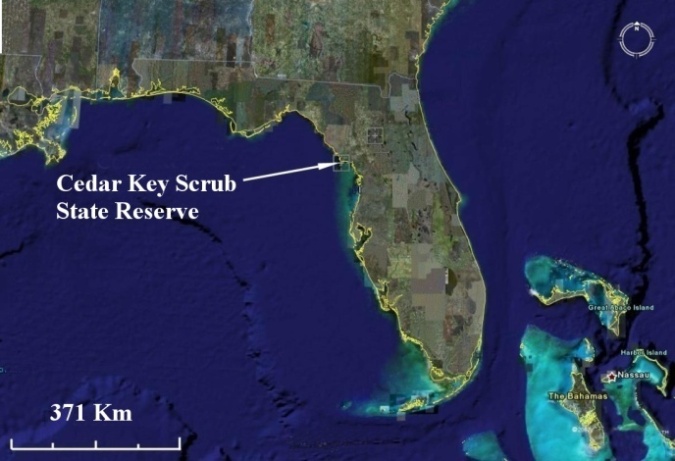 | Figure 1. Location of Cedar Key Scrub State Reserve in Florida |
2.2. Study Design
- The study of literature, the desire to better understand the role of refugia in survival and as a source of reoccupation of burned habitat, and the effect of fire in survival brought the following research hypotheses: (1) Florida mouse, cotton rat, cotton mice, and golden mouse used the vegetation surrounding wetlands next to burned sites as temporary refugia after prescribed burning, (2) Florida mouse, cotton rat, cotton mice, and golden mouse returned to burned sites after plant communities provided at least 50% of vegetation cover, and (3) prescribed burning did not have a negative effect on the survival probability of the Florida mouse, cotton rat, cotton mice, and golden mouse.A quasi-experimental design was planned with two treatment sites (5C and 2M) and two control sites (5A and 5D) not selected at random to test the research hypotheses. Sites 5C, 5A, and 5D were separated by approximately 635 m and they were approximately 2.3 km apart from 2M. The park manager planned to burn long-unburned scrubby flatwoods in the reserve, and we visited them to do the selection. We chose four sites with similar characteristics regarding soil, slope, fuel characteristics, plant species structure and composition, fire history, a wetland next to them, and no mechanical treatment. A site analysis determined that treatment and control sites were ecologically similar [36]. The design included trapping and vegetation sampling in all sites and in the vegetation surrounding wetlands next to all sites before and after prescribed burning. Trapping allowed detection of movements of small mammals in sites, in the vegetation surrounding wetlands, between sites and wetlands, and to obtain the capture/recapture data to estimate survival probabilities. Vegetation sampling enabled quantifies vegetation cover. Based on the results mentioned in the introduction and the natural history of the four species, I predicted that: (1) the vegetation surrounding wetlands next to burned sites would be used as refugia after prescribed burning, (2) small mammals would use these refugia until the vegetation in the burned sites recuperated at least 50% cover of preburn values, and (3) the survival probability of the four species was not negatively affected by prescribed burning.
2.3. Trapping Methods
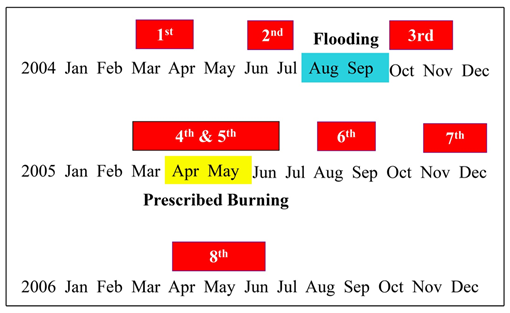
- Four 10x10 grids were used for capturing, marking, and recapturing mice. Grids were installed in the scrubby flatwoods of treatment and control sites. Each grid had 100 standard-sized Sherman Live Traps (7.6 cm x 8.9 cm x 22.9 cm) arranged in 10 lines with 10 trapping stations each and 15 m between trapping stations. In addition, two trap lines with 10 traps each (15 m between traps) were placed between each grid and the wetland next to it. Trap lines were in the vegetation near the border of each wetland to detect mice moving from the grids. Each trap was baited with a 50-50 mix of crimped oats and sunflower seeds, and polyester was used as nesting material during periods of cool weather. Palmetto fronds were used to shade and insulate traps. Traps were checked at sunrise and midafternoon because of the diurnal activity of cotton rat, and traps were left set at all times during the trapping session.Four trapping sessions were conducted before and after prescribed burning (Figure 2) and were planned one session every three months. A session consisted in trapping simultaneously in pair of grids, including one treatment and one control grid (5C-5A and 2M-5D). All sessions were performed up to a 5 days per site sequence to avoid possible loss in body mass associated with capture [37]. The 3rd trapping session started in October and not in September because three hurricanes hit Cedar Key. The 4th session began in March rather than in January due to low temperature (<50 F), and it was completed before applying prescribed burning to 5C on 04/21/05. The 5th session started in 5C on 04/26/05, prescribed burning took place in 2M on 05/18/05, and trapping began in 2M on 05/23/05. Each session lasted 20 days for four sites, but closing traps because of predators (3-5 days/site after the 1st or 2nd day of trapping) extended each trapping session up to 40 days. The exception was the 8th trapping session. It was supposed to start in February 2006, just nine months after burning, but it was cancelled because of the low temperature and rescheduled for April. This session lasted more than two months because trapping for predators was implemented. In treatment and control sites, pretreatment data were collected from 03/02/04 to 04/20/05, and posttreatment data from 04/26/05 to 07/19/06.
2.4. Vegetation Sampling and Prescribed Burning
- Before prescribed burning, vegetation sampling was carried out in treatment and control sites from January to March 2005. Burning reduced 100% the herb and shrub cover on the ground and top-killed the woody vegetation in more than 95% in treatment sites. After prescribed burning, sampling was conducted at interval of three months from May 2005 until April 2006. Vegetation cover was sampled by placing a quadrat (4 m2) on 50 points of the trapping grid selected by a stratified random sampling [38]. Shrub (<1.5 m tall) cover was quantified in cm along a 2 m line that intercepted the center point of two opposite sides of each quadrat.
2.5. Data Analysis
- Due to the small sample size of the capture-recapture dataset, data analysis was only carried out for the Florida mouse and the cotton rat. I combined the data from control and treatment sites for each species and evaluated the effect of prescribed burning on the survival probability by using information-theoretic model selection and inference framework [39]. In addition, I used the program MARK 4.3 [40] for testing lack of fit and for estimating the survival and recapture probabilities by using the Cormack-Jolly-Seber model [41, 42, 43]. The candidate model set consisted of 16 models. The survival probability (phi) was considered constant (.), time dependent (t), group dependent (g; treatment – control), and dependent of the interaction group and time (g*t). The recapture probability (p) was also considered under the same conditions, and the combination of all these possibilities added up 16 models. This set of models was used as the candidate model set. I fitted these models to the data by using the “predefined model” option in MARK and carried out the Goodness of Fit (GOF) test for the full time-dependent model phi(g*t) p(g*t). The GOF test was done by using Bootstrap and Release methods. The Bootstrap method provides two ways to estimate c-hat: the deviance and the c-hat method. I followed Cooch and White’s [44] recommendations regarding which c-hat to choose between Bootstrap and Release. These authors suggest to choose the largest c-hat value in the interval 1 < c-hat < 3 in order to make the model selection more conservative. This c-hat means over dispersion of the dataset, and an adjustment for lack of fit is needed by using this particular c-hat value. The Bootstrap deviance method provided the greatest c-hat for the Florida mouse and the cotton rat. Hence, the full-time dependent model and the candidate model set were adjusted to these c-hats. Since the dataset was small, MARK calculated the corrected Akaike’s Information Criterion (AIC) or AICc. Furthermore, since the candidate model set was adjusted by using a c-hat value, MARK displayed the quasilikelihood AICc or QAICc values.To compare and select models, the following steps were carried out. First, selecting the most parsimonious model in the candidate model set by using QAICc. Models were considered well supported if ∆QAICc ≤ 2. Second, in the Florida mouse, the most parsimonious model was phi (t) p(.), and the second and third best models were phi(t) p(g) and phi(t) p(t), respectively. In cotton rat, the most parsimonious model was phi (t) p(.) and the second one was phi(t) p(g). The survival probability (phi) was only time dependent, and the probability of recapture (p) was constant (.), time dependent (t), and group dependent (g). Therefore, I considered building other models to test flood and prescribed burning effects based on these preliminary results.Third, adding models phi (Flood + Fire) p(., t, g) for the Florida mouse and phi (Flood + Fire) p(., g) for the cotton rat. Flood and prescribed burning (Fire) were time dependent variables and their additive effects were modelled with the probability of recapture constant, time dependent, and group dependent. These combinations resulted in nine models for Florida mouse and six models for cotton rat that were added to the candidate model set of 16 models for comparison purposes. In addition, because models phi(Flood + Fire) p(t) and phi(Flood) p(t) had the greatest support in the data in the Florida mouse, and p(t) was present in these two models, I added models phi(Flood + Fire) p(Flood+Fire), phi(Flood + Fire) p(Flood), and phi(Flood + Fire) p(Fire) to analyze the effect of the time-dependent covariates Flood and Fire in the recapture probability. Then, the most parsimonious model was selected out of 28 and 22 models for Florida mouse and cotton rat, respectively. The covariate Flood was included in the survival analysis of these two species because three hurricanes hit Cedar Key and partially flooded treatment and control sites. No previously marked mice were recaptured after flooding. Since, this covariate had a strong influence in the survival probability in both species; its additive effect with Fire was modeled by adding linear constraints to MARK. The basic sequence of steps of building design matrices followed Cooch and White [44]. Recording two natural disturbances was a lucky event and modeling them makes this study unique. Fourth, checking for the number of real parameters and adjusting them. Even though MARK estimates the number of parameters, the model structure determines the number of parameters that are theoretically estimable. However, if the sample size is small, not all theoretically estimable parameter can be estimated. In addition, when survival and recapture are time dependent, the terminal parameter is not individually identifiable [45]. Since the sample size for the mark-recapture data is small in this study, I manually checked if the number of estimable parameters indicated by MARK matched the number of β parameters theoretically estimable for a particular model. If they did not match (one or more β parameters were not estimated), I manually adjusted the number of parameters to the theoretical number.Fifth, I estimated survival parameters by using modeling averaging. Reporting survival estimates from the most parsimonious model ignored model uncertainty. For this reason, survival estimates were reported utilizing modeling averaging from the entire model set, which weights parameter estimates using normalized QAICc model weights [46]. Finally, survival estimates from both the most parsimonious model and model averaging were plotted. The purpose of this comparison was to show similarities or dissimilarities between estimated parameters from both approaches.
3. Results
3.1. Number of Captured Individuals in Treatment and Control Sites
- A total of 182 individuals were marked and recaptured 426 times in 29,340 trapping nights in treatment and control sites. Table 1 presents the number of recaptures, new individuals, and total captured individuals per trapping session in treatment and control sites. Considering the total individuals captured per species in treatment sites, all species were captured in low numbers (one to nine individuals) at the beginning of the study (1st-3rd trapping session). Of 39 individuals marked in the first two trapping sessions, none were recaptured after the hurricanes during the 3rd trapping session and during the additional trapping effort carried out in upper grounds. Most likely, they died. After the 3rd trapping session, the number of individuals of Florida mouse and cotton rat increased from 13 to 21 individuals and from 13 to 34 individuals, respectively, even after prescribed burning. The number of captured individuals of cotton mice was stable (four to seven individuals) and only one golden mouse was recaptured through time.
3.2. Number of Captured Individuals in Scrubs and Wetlands
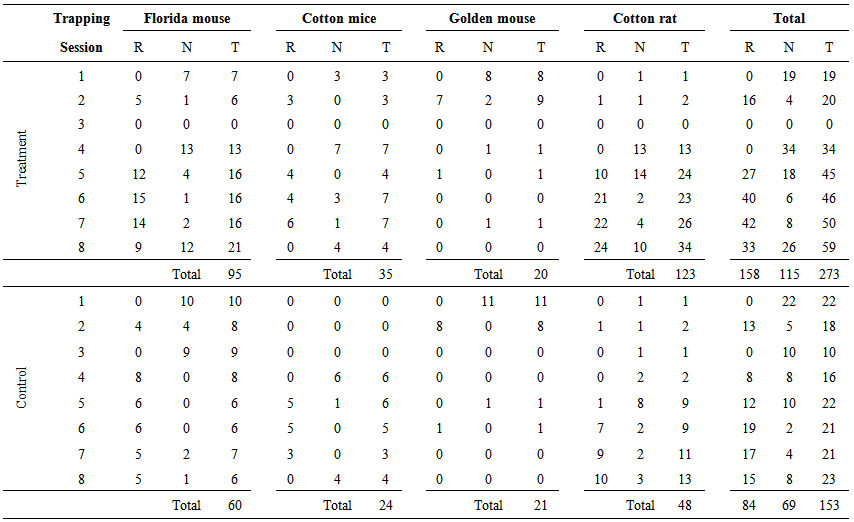
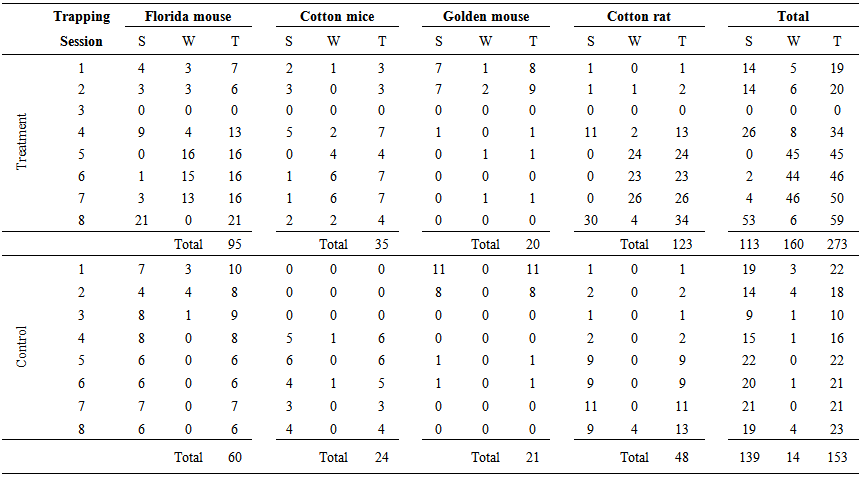
- Table 2 shows the number of captured individuals per species in scrubby flatwoods (scrubs hereafter) and in the vegetation surrounding wetlands (wetlands henceforth) per trapping session in treatment and control sites. In treatment sites and before prescribed burning (1st - 4th trapping sessions), I captured 73 individuals in both scrub and wetlands, but 54 (74.0%) were captured in scrubs. During the 4th trapping session after the hurricanes, 26 (76.5%) of 34 new marked individuals were captured in scrubs. Of these 26 mice, 22 (84.6%) were recaptured in wetlands during the 5th trapping session right after prescribed burning. In addition, during the 5th trapping session in wetlands, five mice previously trapped in wetlands were recaptured and 18 new individuals were captured. Therefore, marked mice moved to or stayed in wetlands after prescribed burning and new individuals preferred wetlands rather than scrubs. During the 6th and 7th trapping sessions, 90 individuals were captured in wetlands and six in scrubs. The 90 individuals included mainly previously marked individuals (82) rather than new ones (8). The six individuals found in scrubs corresponded to one Florida mouse marked in the 6th and recaptured in the 7th trapping session, two new Florida mouse marked in the 7th session, and one cotton mouse recaptured in the 6th and 7th trapping sessions. Of 46 mice captured in wetlands during the 7th trapping session, 31(67.4%) were recaptured in scrubs during the 8th trapping session. During this last trapping session, one year after prescribed burning, I captured 59 individuals in both scrubs and wetlands and 53 (89.8%) were trapped in scrubs. The 53 individuals included 31 recaptured mice from the 7th trapping session and 22 new individuals. The six individuals captured in wetlands were new. During all trapping sessions in control sites, 153 individuals were captured, from which 139 (90.8%) mice were captured / recaptured only in scrubs. Therefore, mice returned to the scrubs in treatment sites after at least 11 months (May 2005-March 2006) following prescribed burning. These results supported the 1st research hypothesis, but only for the Florida mouse and the cotton rat. The results obtained for the cotton mouse and the golden mouse are not conclusive because of the small sample size.
3.3. Flood and Fire Effects on the Florida Mouse
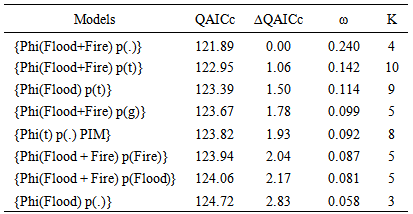
- The additive effect Flood + Fire and Flood had an effect on the survival probability of the Florida mouse. Table 4 presents the top eight of 28 models fitted, adjusted to c-hat = 1.1387, and corrected for the number of parameters. As shown in this table, the top model phi (Flood + Fire) p(.) had 24.0% support in the data, and there was not enough evidence to indicate that this model was different from the 2nd to the 5th model because ∆QAICc < 2.0. However, the additive effect of Flood + Fire and the covariate Flood had an influence on the survival probability of Florida mouse because this set of four models was supported by 59.5% of the data, and phi was dependent of Flood + Fire in three models. Even though the covariate Fire by itself did not have support in the data, the effect of fire could be seen by comparing model phi (Flood + Fire) p(.) with phi (Flood) p(.). Delta QAICc = 2.83, and this was the Fire effect. The effect of Flood could be noted by comparing phi (Flood + Fire) p(.) with phi (Fire) p(.) (∆QAICc = 30.11). This was the Flood effect. The time-dependent covariate Flood and Fire did not have an important influence in the recapture probability because these models had very little support in the data (< 8.7%).
|
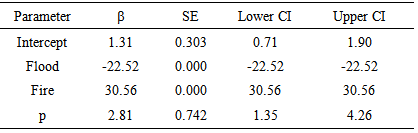
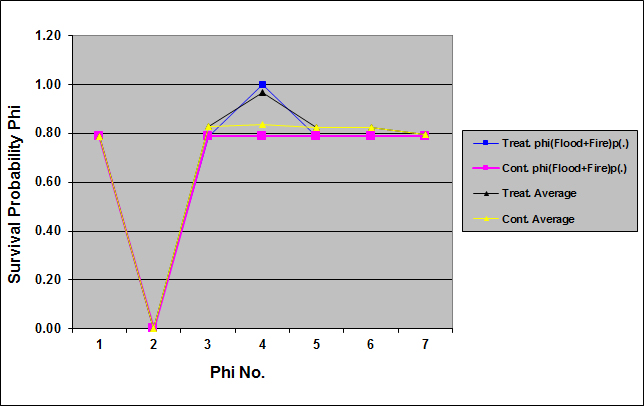 | Figure 3. Survival probabilities for the Florida mouse calculated by model phi(Flood + Fire ) p(.) and by model averaging for the set of 28 models in treatment (Treat.) and control (Cont.) sites in Cedar Key Scrub State Reserve, Cedar Key, Florida, between 2004 and 2006 (chat =1.1387) |
|
3.4. Flood and Fire Effects on the Cotton Rat
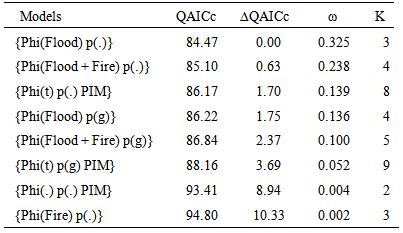
- The covariate Flood and the additive effect Flood + Fire had an influence on the survival probability of cotton rat. Table 6 shows the top eight of 22 models fitted, adjusted to c-hat = 1.5694, and corrected for the number of parameters. As can be seen in this table, the top model, phi (Flood) p(.) had 32.5% support in the data, but it was no different from the 2nd to the 4th model because ∆QAICc < 2.0. Of the remaining 18 models, phi(Flood + Fire) p(g) and phi(t) p(g) had 10.0% and 5.2% support in the data, respectively, but the rest of the models had a support ≤ 0.40% in the data. In addition, there were considerable evidences for a real difference between phi(Flood) p(.) and the 5th and 6th models because ∆QAICc > 2.0. The first four models had 83.8% supports in the data and it is conformed mainly by the covariates Flood and Flood + Fire. Thus, only models with Flood and Flood + Fire on the apparent survival rate had a substantial support in the data. Therefore, these covariates had an effect of the survival probability of the cotton rat.
|
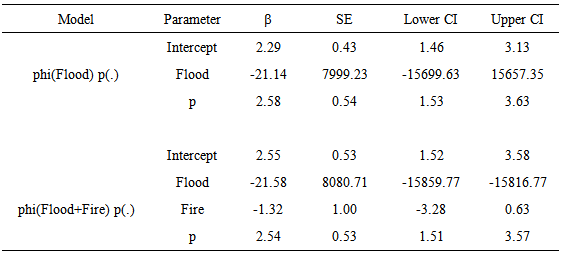
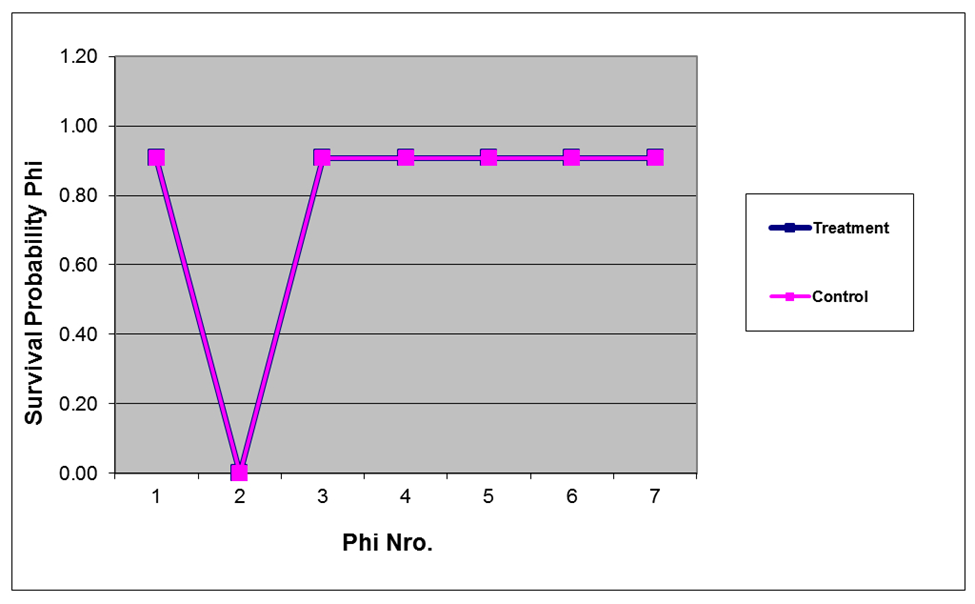 | Figure 4. Survival probabilities for the cotton rat estimated by model phi(Flood) p(.) in treatment and control sites in Cedar Key Scrub State Reserve, Cedar Key, Florida, between 2004 and 2006 (chat =1.5694) |
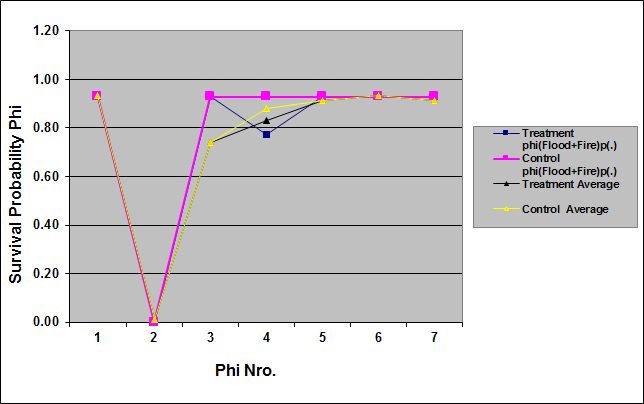 | Figure 5. Survival probabilities for the cotton rat estimated by model phi(Flood + Fire) p(.) and by model averaging for the set of 22 models in treatment and control sites in Cedar Key Scrub State Reserve, Cedar Key, Florida, between 2004 and 2006 (chat =1.5694) |
|
4. Discussion
4.1. Flooding Effect
- Hurricanes Charley, Frances, and Jeanne hit Cedar Key and all sites were flooded. The approximate percentage of grids covered by water was as follows: 5C = 5A =50%, 2M = 60%, and 5D = 30%. All sites stayed flooded for at least two weeks and mice only had two options, move to higher ground or die. I did not capture mice in upper grounds inside/outside the grids during the extra trapping effort. Most likely, 79 mice marked during the first two trapping sessions died in treatment and control sites.Flooding negatively affected the small mammal community independently of their life form. Even though the effect of flooding on the survival probabilities of Florida mouse and cotton rat was not conclusive statistically, the effect of flooding upon terrestrial species was expected to be detrimental. Arboreal species such as golden mouse should have a better possibility to tolerate this type of disturbance, but it did not because flooding lasted at least two weeks in all sites. No previous survival analysis of the effect of flooding on small mammals was found, but some references support the negative effect of a long period of flooding. No detrimental effect upon the population of cotton mice and golden mouse in Texas was recorded when flooding occurred up to 8 days. Flooding for a 3 weeks period caused a marked decrease in the populations. This probably happened because individuals tended to remain within established home range even during long periods of flooding [47]. White-footed mice (Peromyscus leucopus) completely disappeared from floodplain plots after severe flooding [48, 49]. White-footed mice, mountain vole (Microtus montanus), and kangaroo rat (Dipodomys ordii) generally experience habitat inundation as catastrophic [50].
4.2. Burrows as Refugia during Prescribed Burning
- Most likely, prescribed fire did not cause mortality of the small mammal community in CKSSR because mice hid in burrows. Fire intensity was high enough in treatment sites to remove all above ground vegetation and no evidence of mice mortality was found. Most likely, mice hid in 28 burrows in each treatment site during prescribed burning and moved to the vegetation surrounding wetlands after it. Out of 26 individuals of Florida mouse, cotton rat, cotton mice, and golden mouse marked in scrubs during the 4th trapping session in treatment sites, 22 were recaptured in wetlands after prescribed burning during the 5th trapping session. Burrows increased survivorship because of the insulating characteristic of the soil. Some studies have shown that temperatures higher than 100 °C at the surface of the ground decline in the first 2.5 cm of soil depth to temperatures around 20-30 °C in longleaf pine in south-eastern USA [51], in Australia eucalypt forest [52], in California Chaparral [53], and in heavy slash fuels after logging a forest [54]. One reason for poor penetration of heat is that convective heat is transferred upward. Burrows as refugia for small mammals during prescribed fire have been documented in the literature [2, 55, 56, 57, 58, 59, 60, 61].
4.3. Prescribed Burning Effect
- The lack of a negative prescribed burning effect found in this study has also been cited in the literature. Seven studies have reported survival analysis and five of them indicated that prescribed burning did not have any negative effect. A population of dusky-footed woodrats (Neotoma fuscipes) fluctuated from 1993 to 2001 and decreased after prescribed fire in California oak woodlands. Apparently, juvenile survival was the cause of the population fluctuation. Prescribed fire did not have any support in the data [25]. Densities of deer mouse and lodgepole chipmunk (Neotomias speciosus) are more influenced by year effect than prescribed burning in a mixed conifer forest in Sequoia National Park, California. Fire by itself had less than 0.01% support in the data [26]. The effect of fire was not significant on the survival probabilities of Pinyon deermouse (Peromyscus truei), brush deermouse (P. boylii), and California pocket mouse (Chaetodipus californicus) in a mixed blue oak-coast live oak in California [28]. Forest thinning increased densities of deer mouse, gray-collared chipmunks (Tamias cinereicollis), golden-mantled ground squirrels (Spermophilus lateralis), and Mexican woodrats (Neotoma mexicana) in ponderosa pine in Coconino National Forest, Arizona. But, the combination of thinning and frequent prescribed fire might have reduced small mammal densities [23]. Survival of the cotton mouse was similar across all treatments and controls sites after a prescribed burning in a longleaf pine (Pinus palustris) ecosystem in Ichauway, Georgia [27]. In the same study area, a winter prescribed fire caused a short-term reduction in survival of cotton rats within two sites subjected to mammal predator control. This reduction occurred because the majority of cotton rats did not emigrate and exposed to avian predators due to the reduction of cover and food [22]. However, another study conducted in Ichauway found out that prescribed fire increased apparent monthly survival of cotton mice and cotton rat relative to their respective base models [24].
4.4. Population Increases/Decreases after Prescribed Burning
- There was an increase in the number of individuals of Florida mouse and cotton rat after prescribed burning. This increase was clearly identified after comparing treatment and control sites (Table 1). A 100% reduction of the herb and shrub vegetation in treatment sites after prescribed burning forced both species to move to the vegetation surrounding wetlands. However, although cotton mice and the only individual of golden mouse did the same, the number of individuals did not increase through time. Therefore, prescribed burning was the stimulus to move, but the increase in number of individuals in the vegetation surrounding wetlands had to deal with other factors such as immigration, food/space availability, and competition. I only had data for the first factor. The increase in the number of individuals was due to new adult individuals. Maybe, the burned area attracted these mice but they had to seek refuge in the vegetation surrounding wetlands because of the lack of cover and food in the burned area. The lack of increase in the number of individuals of cotton mice was surprising, but the drastic decline in the number of individuals of golden mouse was expected. The majority of the studies have shown a neutral or positive response of the genus Peromyscus to prescribe fire in the burned area [27, 56, 62]. Only one cotton mouse was captured in one burned area and six in wetlands between the 5th and the 7th trapping sessions in Cedar Key (Table 2). Most likely, this was a consequence of lack of enough cover and food during the regrowth of vegetation in treatment sites and the vegetation surrounding wetlands was too dense for this species. The decline in the number of individuals of golden mouse was predictable because of its arboreal life form and the combined effect of flooding and prescribed burning.
4.5. Habitat Selection: Immigration, Emigration, and Returning to Burned Areas
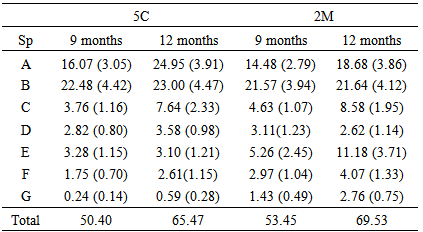
- In general, prescribed burning affects small mammals mainly through the way it affects their habitats. Direct effects such as injury, mortality, and movement (immigration and emigration) might be the short-term population responses. Indirect effects through habitat alteration could influence long-term responses such as feeding, movement, reproduction, and availability of refugia [2]. In both circumstances, immigration and emigration have an important role in population demography, food availability, reproduction, and recolonization of the burned areas. Immigration might occur because burned areas attract small mammals; however, emigration could also take place if there is insufficient food and cover in the burned area. Characteristics of an animal species such as mobility and particular food and cover requirements will determine its ability to re invade a burned site [63]. The length of time before these species return to burned sites depends on how much fire altered the habitat structure and food supply [2]. The last three sentences summarize and explain what Florida mouse and cotton rat experienced at CKSSR. I do not make statements for the cotton mouse and the golden mouse because of the low number of captures.Although I only trapped a few new individuals in burned sites following prescribed burning, the literature has reported immigration to burned areas. Between the 5th and the 7th trapping sessions, only three new Florida mouse and one new cotton mouse were captured in burned sites. Probably, other new individuals were attracted by the burned area and moved to wetlands looking for cover and food. Odors from burned areas might stimulate immigration of deer mouse from suboptimal habitats in Kansas tallgrass prairie [64]. Deer mouse invaded a slash burned area immediately after prescribed fire in jack pine in northeastern Minnesota [19]. The number of resident individuals of Florida mouse in burned areas of longleaf/turkey oak habitat in Ordway-Swisher Preserve was higher than in the unburned ones [58, 65].Emigration to unburned sites has also been reported in the literature. The lack of prescribed burning effect on Florida mouse and cotton rat was because they emigrated to and used the vegetation surrounding wetlands as refugia. Statistically, I could not draw any conclusion regarding fire effect on the Florida mouse and it was not significant for the cotton rat. However, even though the dataset was small, it was good enough to demonstrate that these two species emigrated to wetlands where they found cover and food. Although Florida mouse in the current study moved out of burned areas, no individuals of Florida mouse leaved burned areas except one after prescribed burning in Ordway-Swisher Preserve [58]. This was probably due to a patchy burn in the Ordway sandhills in comparison with a more continuous and high intensity burn in the CKSSR scrub. Cotton rat moved from the burned to the un-burned site following prescribed fire near Gainesville, Florida [7]. Red squirrel (Tamiasciurus hudsonicus), voles (Microtus spp), and flying squirrel (Glaucomys spp.) emigrated from recent burned areas [1]. Townsend’s chipmunk (Neotamias townsendii) and dusky-footed woodrat were not abundant in recently burned areas in chaparral brushlands [66]. Even though species emigrate from burned sites, most of the time they return to the same burned site they moved away from months ago.Florida mouse and cotton rat returned to the burned sites in CKSSR after at least 11 months. This amount of time appears to be a long time for a mouse to live and survive. Out of 49 Florida mice (19 individuals) and cotton rats (30 individuals) captured between the 5th and the 7th trapping sessions, 33 (67.3%) individuals (nine Florida mouse and 24 cotton rats) were recaptured again in scrub in the 8th trapping session. The survival curve for both species was high (Figures 3 and 4), and the amount of time involved between the 5th and the 8th trapping session was 373 days for 5C and 403 d for 2M. Are Florida mouse and cotton rat long lived species? Jones [58] at Ordway Swisher Preserve found that 8.6% of all marked mice (225 individuals) were present for 360 days or more. Of these mice, half were females first marked as juveniles, and most of the males were first marked as subadults and adults. The longevity records were 649 days for females and 920 days for males. Layne [17] reported two cotton rat females, originally trapped as subadults, recorded on the study area during the entire 14-month period. A juvenile female, an adult male, and a juvenile male were first captured in October 1960 and they were recaptured 10 months later in July 1961. Florida mouse and cotton rat in CKSSR returned to the burned sites after they found cover and food. It is surprising that both species returned approximately at least 11 months later. But, it is not as surprising when we consider the phenology of the plant species. Acorns of myrtle oak, Chapman oak, and sand live oak developed surrounding wetlands in September and production ended in December 2005. Thus, Florida mouse and cotton rat had food to stay in the vegetation surrounding wetlands. In the burned sites 5C and 2M, the percentage of shrub cover at 11 months after prescribed burning was not quantified, but at 12 months was 65.5% and 69.5%, respectively (see Table 3). Blueberry was the first plant to develop flowers in March and fruits in April 2006, and Florida mouse and cotton rat returned to scrubs during this event. The relationship between the amount of cover and mice returning to burned areas has been reported in the literature. Layne [17] was the first to report that the return of cotton rat and eastern harvest mouse to a burned area appeared to be correlated with redevelopment of the ground cover in slash/longleaf pine habitat in north-central Florida. West [67] indicated that northern red-backed voles avoided a burned area in black spruce for one year and established a resident population in the fourth postfire year, which it was the first year of berry production in central Alaska. Kirkland et al. [18] found that the rapid recovery of small mammal populations was explained by the fast regrowth of ground cover, particularly of blueberry. Schwilk and Keeley [6] carried out a patchy burn in a California chaparral and coastal sage scrub. Unburned patches acted as refugia and allowed small mammals colonized severely burned sites during the first six months after prescribed fire. Ford et al., [68] also used the link between small mammals and regrowth of the vegetation as the explanation of the population recovery in the study conducted in Southern Appalachian, North Carolina. Kirchner et al., [69] reported that relative abundances of cotton rat and pigmy mice (Baiomys taylori) returned to preburn levels in burned sections after grasses and forbs cover regrew in eight months at tallgrass blackland prairie in Texas.
4.6. Implications for Wildlife Management and Ecosystem Restoration
- Because so many species rely on small mammals as prey and for ecosystem services (seed dispersal and soil conditioning), it is relevant to consider small mammals’ responses to prescribed burning [28]. This and other studies support the hypothesis that prescribed burning does not negatively affect the survival probability of small mammals. Therefore, prescribed burning can be safely applied as a management tool for restoration purposes in scrubby flatwoods, but the prescription should consider refugia for small mammals. This is not the norm, at least in Florida. I suggest that if the area to be burned is surrounded by habitats included in the prescribed burning plan during the same season, the vegetation next to wetlands and to the area to be burned should not be included in the prescribed burning plan. The vegetation next to wetlands and to the area to be burned will be the only refugia available to wildlife. If the area to be burned has surrounding habitats not included in the prescribed burning plan, land managers could burn the vegetation next to wetlands. Another possibility is to burn the vegetation next to wetlands after giving the burned area enough time (at least one year) to re-establish cover and food. Prescribed fire should be limited to the spring and early summer and applied to only a portion of the total area [70].
5. Conclusions
- Prescribed burning did not negatively affect the survival probability of the Florida mouse and the cotton rat in scrubby flatwoods because they used the vegetation surrounding wetlands as refugia for at least 11 months. Emigration to refugia occurred because there was no cover and food in the burned sites after a high intensity prescribed burning. The recolonization of the burned sites took place in or after March 2006 because of the regrowth of the shrub cover (>50%) and the production of flowers and fruits. The use of prescribed burning as a management tool in the scrubby flatwoods should include identification and protection of refugia for small mammals because of their critical role as prey species and in ecosystem services. The vegetation surrounding wetlands should be burned only if other refugia are offered or after burned sites provide cover and food.
ACKNOWLEDGEMENTS
- The Department of Wildlife Ecology and Conservation and the Department of Environmental Protection provided the financial aid and logistic needed for carrying out fieldwork. Florida Fish and Wildlife Conservation Commission provided a GPS unit and participated during prescribed burning. Dr. George Tanner was my adviser during my Ph.D. dissertation, and I am very grateful for all the support, help, assistance, and guidance. Dr. James Waddle and Dr. Arpat Ozul assisted with the MARK program. Jeff DiMaggio and David Hoyt helped to cut the dense scrub vegetation for making four trapping grids during three months and provided logistic help during fieldwork. Jason Hall shared the field vehicle assigned to him. Dr. Susan Carr and Zachariah Welch lent PC-ORD software and books.
References
| [1] | Ream, C. H., 1981, The effects of fire and other disturbances on small mammals and their predators: an annotated bibliography, GTR-INT-106, U.S. Department of Agriculture, Forest Service, Ogden, UT. 55 p. |
| [2] | Smith, J. K., 2000, Wildland fire in ecosystems: effects of fire on fauna. GTR-RMRS-GRT-42-I U.S. Department of Agriculture, Forest Service. Ogden, UT, 83 p. |
| [3] | Goatcher, B. L., 1990, A preliminary investigation of the importance of stream-terrace hardwood forests as refuge for small mammals to escape fires, M.S. Thesis, Louisiana State University, Baton Rouge, Louisiana, 53 p. |
| [4] | Blanchard, V. J., 1991, Stream-terrace hardwood forest as refuge for small mammals when adjacent pine forest is burned, M.S. Thesis, Louisiana State University, Baton Rouge, Louisiana. 89 p. |
| [5] | McGee, J. M., 1982, Small mammal populations in an unburned and early fire successional sagebrush community, J. Range Manage., 35, 177-180. |
| [6] | Schwilk, D. W., and Keeley, J. E., 1998, Rodent populations after a large wildfire in California chaparral and coastal sage scrub, Southwest Nat., 43, 480-483. |
| [7] | Arata, A. A., 1959, Effects of burning on vegetation and rodent populations in a longleaf pine turkey oak association in north central Florida, Q. J. of Fla. Acad. Sci., 22, 94-104. |
| [8] | Blankenship, D. J., 1982, Influence of prescribed burning on small mammals in Cuyamaca Rancho State Park, California, Pp. 587, In: Conrad, C. E., and Oechel W. C. (eds.), Proceedings of the Symposium of Dynamics and Management of Mediterranean Type Ecosystems; 1871 Jun 22-26; Sand Diego, CA. GTR-PSW-58, U.S. Department of Agriculture, Forest Service, Berkeley, California. |
| [9] | Forde, J. D., Sloan, N. F., and Shown, D. A., 1984, Grassland habitat management using prescribed burning in Wind Cave National Park, South Dakota, Prairie Nat., 16, 97-110. |
| [10] | Komarek, E. V., 1965, Fire Ecology – grassland and man. Proceedings of the Annual Tall Timbers Fire Ecology Conference, 4, 169-220. |
| [11] | Komarek, E. V., 1969, Fire and animal behavior. Proceedings of the Annual Tall Timbers Fire Ecology Conference, 9, 160-207. |
| [12] | Lee, A. K., 1963, The adaptations to arid environments in woodrats of the genus Neotoma. Univ. Calif. Publ. Zool., 64, 57-96. |
| [13] | Odum, E. P., Pomeroy, S. E., Dickinson III, J. C., and Hutcheson, K.., 1973, Effects of late winter litter burn on the composition, productivity, and diversity of a 4-year old fallow-field in Georgia, Proceedings Annual Tall Timbers Fire Ecology Conference, 13, 399-419 |
| [14] | Tevis, L. Jr., 1956, Effect of a slash burn on forest mice, J. Wildl. Manage., 20, 405-409. |
| [15] | Wirtz, W. O., 1977, Vertebrate post-fire succession, Pp. 46-57. In: Mooney, H. A., and Conrad, C. E. (eds.), Proceedings of the Symposium on Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems, 1977 Aug 1-5; Palo Alto, CA. GTR-WO-3, U.S. Department of Agriculture, Forest Service, Washington, D.C.. |
| [16] | Wirtz, W. O., Hoekman, D., Muhm, J. R., and Souza, S. L., 1988, Postfire rodent succession following prescribed fire in southern California chaparral, Pp. 333-339, In: Szaro, P. C. Severson, K. E., and Patton, D. R. (eds.), Proceedings of Symposium about Management of Amphibians, Reptiles, and Small Mammals in North America, GTR-RM-166, U.S. Department of Agriculture, Forest Service. Ft. Collins, Colorado. |
| [17] | Layne, J. N., 1974, Ecology of small mammals in a flatwoods habitat in north-central Florida, with emphasis on the cotton rat (Sigmodon hispidus), Am. Mus. Novit., 2544, 1-48. |
| [18] | Kirkland Jr., G. L., Snoddy, H. W., and Amsler, R. L., 1996. Impact of fire on small mammals and amphibians in a central Appalachian deciduous forest, Am. Midl. Nat., 135, 253-260. |
| [19] | Ahlgreen, C. E., 1966, Small mammals and reforestation following prescribed burning, J. For., 64, 614-618. |
| [20] | Sullivan, T. P., and Boateng, J. O., 1996, Comparison of small mammal community responses to broadcast burning and herbicide application in cutover forest habitats, Can. J. For. Res., 26, 462-473. |
| [21] | Taylor, D. L., 1981, Effects of prescribed fire on small mammals in the southeastern United States, Pp. 109-120, In: Wood, G. W. (ed.), Prescribed fire and wildlife in southern forest, Belle W. Baruch Forest Sci. Inst. Clemson Univ., Georgetown, South Carolina. |
| [22] | Conner, L. M., Castleberry, S. B., and Derrick, A. M., 2011, Effects of mesopredators and prescribed fire on hispid cotton rat survival and cause-specific mortality, J. Wildl. Manage., 75, 938-944. |
| [23] | Converse, S. J., Block, W. M., and G. C. White, 2006, Small mammal population and habitat responses to forest thinning and prescribed fire, For. Ecol. Manage., 228, 263-273. |
| [24] | Karmacharya, B., Hostetler, J. A., Conner, L. M., Morris, G., and Oli., M. K., 2012, Longleaf pine management practice and their impact on small mammal populations, For. Ecol. Manage., 271, 140-146. |
| [25] | Lee, A. K., and Tietje, W. D., 2005, Dusky-footed woodrat demography and prescribed fire in a California oak woodland, J. Wildl. Manage., 69, 1211-1220. |
| [26] | Monroe, M. E., and Converse S. J., 2006, The effects of early season and late season prescribed fires on small mammals in a Sierra Nevada mixed conifer forest, For. Ecol. Manage., 236, 229-240. |
| [27] | Morris, G., Hostetler, J. A., Oli, M. K., and Conner, L. M., 2011, Effects of predation, fire, and supplemental feeding on populations of two species of Peromyscus mice, J. Mammal., 92, 934-944 . . |
| [28] | Tietje, W. D., Lee, D. E., and Vreeland, J. K., 2008, Survival and abundance of three species of mice in relation to density of shrubs and prescribed fire in understory of an oak woodland in California , Southwestern Nat., 53, 357-369. , Southwestern Nat., 53, 357-369. |
| [29] | Myers, R. L., 1990, Scrub and high pine, Pp. 150-193, In: R. L. Myers and J.J. Ewel, eds., Ecosystems of Florida. University of Presses Florida, Gainesville, 765 pp. |
| [30] | Schmalzer, P. A. 2003, Growth and recovery of oak-saw palmetto scrub through ten years after fire, Natural Areas Journal, 23, 5-13. |
| [31] | Layne, J. N., 1992, The Florida mouse, Pp. 250-264, In: Humphrey, S. R. (ed.), Rare and endangered biota of Florida. Volume I: Mammals. University Press of Florida, Gainesville, Florida. |
| [32] | Stout, I. J., 2001, Rare plants of the Florida scrub, USA, Natural Areas Journal, 21, 50-60. |
| [33] | Layne, J. N., 1990, The Florida mouse, Pp. 1-21, In: Dodd, C. K. Jr. Ashton, R. E., Franz, R., and E. Wester (eds.), Burrow associates of the gopher tortoise, Eight Ann. Mtg. Gopher Tortoise Council. Florida Museum of Natural History, University of Florida, Gainesville, Florida. |
| [34] | DEP, 1998, Cedar Key Scrub State Reserve: Unit Management Plan, Division of Recreation and Parks, Tallahassee, Florida, 136 p. |
| [35] | Morgan, G. L., 1998, Florida mouse (Podomys floridanus) association with gopher tortoise (Gopherus polyphemus) burrows and vegetation characteristics in the Cedar Key Scrub State Reserve, Levy County, Florida, M.S. Thesis, University of Florida, Gainesville, Florida, 79 p. |
| [36] | Silva-Lugo, J. L., and Tanner G. W., 2010, Testing control sites for fire ecology research, J. Torrey Bot. Soc., 137, 263-276. |
| [37] | Slade, N. A, 1991, Loss of body mass associated with capture of Sigmodon and Microtus from Northeastern Kansas, J. Mammal., 72, 171-176. |
| [38] | Krebs, Ch. J., 1999. Ecological methodology, 2nd ed., Addison Wesley Longman, California. |
| [39] | Burnham, K. P., and Anderson D. R., 2002, Model selection and multimodel inference: A Practical Information-Theoretic Approach, Springer, New York. |
| [40] | White, G., and Burnham, K. P., 1999, Program MARK. [Online] Available:http://welcome.warnercnr.colostate.edu/~gwhite/mark/mark.htm |
| [41] | Cormack, R. M., 1964, Estimates of survival from the sightings of marked animals, Biom., 51, 429-438. |
| [42] | Jolly, G. M., 1965, Explicit estimates from capture-recapture data with both death and immigration: stochastic model, Biom., 52, 225-247. |
| [43] | Seber, G. A., 1965, A note on the multiple recapture census, Biom., 52, 249-259. |
| [44] | Cooch, E. and White, G., 2013, Program MARK: A Gentle Introduction. [Online]. Available:http://www.phidot.org/software/mark/docs/book/ |
| [45] | Lebreton, J. D., Burham, K. P., Clobert J., and Anderson, D. R.., 1992, Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies, Ecol. Monogr., 62, 67-118. |
| [46] | Anderson, D. R., 2008, Model based inference in the life sciences: a primer on evidence, Springer, New York. |
| [47] | McCarley, W. H., 1959, Fluctuations and structure of Peromyscus gossypinus in eastern Texas, J. Mammal., 35, 526-532. |
| [48] | Blair, W. F., 1939, Some observed effects of stream-valley flooding on mammalian populations in eastern Oklahoma, J. Mammal., 20, 304-306. |
| [49] | Turner, R. W., 1966, Effects of flooding on the mouse Peromyscus leucopus, Trans. Ill. State Acad. Sci., 59, 390-391. |
| [50] | Andersen, D. C., Wilson, K. R., Miller M. S., and Falck, M., 2000, Movement patterns of riparian small mammals during predictable floodplain inundation, J. Mammal., 81, 1087-1099. |
| [51] | Heyward, F., 1938, Soil temperatures during forest fires in the Longleaf Pine region, J. For., 36, 478-491. |
| [52] | Beadle, N. C. W., 1940, Soil temperatures during forest fires and their effect on the survival of vegetation, J. Ecol., 28,180-192. |
| [53] | DeBano, L. F., Neary, D. G., and Folliott, P. F., 1998, Fire’s effects on ecosystems, John Wiley and Sons, New York. |
| [54] | Neal, J. L., Wright, E., and Bollen, W. B., 1965. Burning Douglas-fir slash: physical, chemical and microbial effects on the soil, Forest Research Laboratory Research Paper, Corvallis, Oregon State University. |
| [55] | Beck, A. M., and Vogl, R. J., 1972, The effect of spring burning on rodent populations in a brush prairie savanna. J. Mammal., 53, 336-346. |
| [56] | Derrick, A. M., Conner, L. M., and Castheberry, S. B., 2010, Effects of prescribed fire and predator exclusion on refuge selection by Peromyscus gossypinus Le Conte (cotton mouse), Southeast. Nat., 9, 773 –780. |
| [57] | Hedlund, J. D., and Richard, W. H., 1981. Wildfire and the short-term response of small mammals inhabiting a sagebrush-bunchgrass community, Murrelet, 62, 10-14. |
| [58] | Jones, C. A., 1990, Microhabitat use by Podomys floridanus in the high pine lands of Putnam County, Florida. Ph.D. Dissertation, Univ. Florida, Gainesville, Florida, 176 p. |
| [59] | Quinn, R. D. 1979, Effects of fire on small mammals in the chaparral, Cal-Neva Wildl. Trans., 1979, 125-133. |
| [60] | Sullivan, J., 1995, Sigmodon hispidus, In: Fire Effects Information System, U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory. [Online]. Available:http://www.fs.fed.us/database/feis/animals/mammal/sihi/introductory.html |
| [61] | Tester, J. R., 1965, Effects of a controlled burn on small mammals in a Minnesota oak-savanna, Am. Midl. Nat., 74, 240-243. |
| [62] | Jones, C. A., 1992, Review of the effects of fire on Peromyscus and Podomys, Fla. Scientist, 55, 75-84. |
| [63] | Whelan, R. J., 1995, The Ecology of Fire, Cambridge University Press, Cambridge. |
| [64] | Kaufman, D. W., Gurtz, S. K., and Kaufman, G. A., 1988, Movements of the deer mouse in response to prairie fire, Prairie Nat., 20, 225-229. |
| [65] | Jones, C. A., 1989, Fire and the Florida mouse (Podomys floridanus), Fla. Scientist, 52, 19. |
| [66] | Biswell, H. H., 1989, Prescribed burning in California wildlands vegetation management, University of California Press, Berkeley, California. |
| [67] | West, S. D., 1982, Dynamics of colonization and abundance in central Alaskan populations of the northern red-backed vole Clethrionomys rutilus, J. Mammal., 63, 128-143. |
| [68] | Ford, W. M., Laerm, J., McCay, T. S., Menzel M. A., and McGill, D. W., 1999, Effects of a community restoration fire on small mammals and herpetofauna in the southern Appalachians, For. Ecol. Manag., 114, 233-243. |
| [69] | Kirchner, B. N., Green, N. S., Sergeant, D. A., Mink, J. N., and Wilkins, K. T., 2011, Responses of Small Mammals and Vegetation to a Prescribed Burn in a Tallgrass Blackland Prairie, Am. Midl. Nat., 166, 112-125. |
| [70] | Depue, J. R., 2005, Responses of the Florida mouse (Podomys floridanus) to habitat management, M.S. Thesis, University of Central Florida, Orlando, Florida, 67 p. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML