-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2014; 4(3): 119-123
doi:10.5923/j.ije.20140403.03
Habitat Effects on Nutritional Quality of Two Marine Fish Fillets, Tiger Tooth Croaker (Otelithes ruber ); Four Finger Threadfins (Eleutheronema thetradactylium)
Sahar Jalili1, Navid Zoriasatein1, Foad Pour2
1Department fishery, Abadan Branch, Islamic Azad University, Abadan, Iran
2Young research club, Abadan Branch, Islamic Azad University, Abadan, Iran
Correspondence to: Sahar Jalili, Department fishery, Abadan Branch, Islamic Azad University, Abadan, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
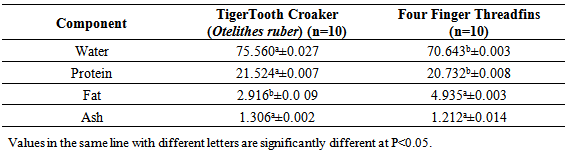
The manuscriptpresents habitat effects on slaughter yield, proximate and fatty acid composition of commercially marine fishes in Persian Gulf, Tiger Tooth Croaker (Otelithes ruber) and Four Finger Threadfins (Eleutheronema thetradactylium). These two species have different habitat Tiger Tooth Croaker (Otelithes ruber) is neriticfish and lives in coastal waters but Four finger threadfins (Eleutheronema thetradactylium) is littoral migratory fish. Two marine fish are carnivorous. Tooth Croaker was characterized by the highest slaughter yield of gutted carcass (755.00±101.06g). Slaughter yield of gutted and deheaded (675.00±101.06g) and deskinned fillet (583.00±100.50g). Four Finger Threadfins had significantly (P≥0.05) higher lipid content than Tiger Tooth Croaker. Four Finger Threadfins is semi-fat fish (2-7%). The crude protein (21.52%) and water (75.56%) contents of Tiger Tooth Croaker have been observed to be higher than Four Finger Threadfins. Total concentration of n-3/n-6 fatty acid is different in Tiger Tooth Croaker and Four Finger Threadfins. Tiger Tooth Croaker carcasses has the high concentration of DHA, (C22:6n-3). The results have indicated Tiger Tooth Croaker is lean fish but it is rich in onega3 fatty acids.
Keywords: Nutrition quality, Habitat, Persian gulf, Carnivorous
Cite this paper: Sahar Jalili, Navid Zoriasatein, Foad Pour, Habitat Effects on Nutritional Quality of Two Marine Fish Fillets, Tiger Tooth Croaker (Otelithes ruber ); Four Finger Threadfins (Eleutheronema thetradactylium), International Journal of Ecosystem, Vol. 4 No. 3, 2014, pp. 119-123. doi: 10.5923/j.ije.20140403.03.
Article Outline
1. Introduction
- Nutritional properties of fish meat and increasing population, the consumption of these resources is greater, (Adeye, 2009). Three marine fishes includes Tiger Tooth Croaker (Otelithes ruber) and Four finger threadfins (Eleutheronema thetradactylium) in Khuzestan coastal waters are the most popular fish in south of Iran with the highest economic value. Tiger Tooth Croaker (Otelithes ruber) is neritic fish and lives in coastal waters. These fish eat tiny crustaceans, fish, shrimp and sea bottom organisms. Four finger threadfins (Eleutheronema thetradactylium) is littoral migratory fish. These fish spend the winter at sea and enter into the small bays and estuaries to reproduce in the warm seasons. Four finger threadfinsfeed plankton.Fish tissue possesses high nutritional value and is therefore a particularly recommended dietary component. Fish tissue is the main source of long-chain poly unsaturated fatty acids n-3 (n-3 LC-PUFA) with beneficial or even therapeutic effects on human health, (Zuirani et al, 2006).The significance of the long chain PUFA has gained attention because of the prevention of human coronary artery disease and important of retina and brain development and also decreasing incidence breast cancer, rheumatoid arthritis, multiple sclerosis and inflammation, (Zhao et al, 2010).In addition, fish tissue protein is characterized by a very desirable composition of amino acids. This tissue is also rich in vitamin A and D. Furthermore, Fish are a good source of micro- and macro-elements (calcium, phosphorus, iodine, selenium, fluorine, and manganese). The chemical composition of fish tissue can be modified by several factors such as feed, development phase, territory, catch season and ontogenic traits, (Zmijewski et al, 2006). Proximate biochemical analysis provides information on the nutritional value of a particular organism used as a source of food, (Ozgoul et al, 2007). Improved knowledge in nutrition has shown how diet can influence human health directly, (simda et al, and 2009).Many studies have been conducted on proximate composition of different fish species in different part of the world (Badii; 2001, Sengor et al., 2003; Celic et al; 2005, Ozogul et al; 2006, Ferreira de Castro et al; 2007, Guler et al; 2008, Ersoy et al., 2010; Dike et al., 2010 Oksuz and Ozlimaz., 2010; Ekin and Bashan., 2010; Ahmed Louly et al; 2011, Sulma and Ogata., 2012).The aim of this study was to evaluate habitat effects on the proximate composition and fatty acid profiles of two selected important marine fish species in Khuzestan coastal waters (Persian Gulf).
2. Material and Methods
2.1. Preparation of Experimental Material
- The experimental fish were obtained in national condition (Khuzestan coastal waters, south of Iran) between March to October 2012. Of each species catch, 10 fish with similar body weights were selected for analysis. The fish were then killed and their body weights (BW±1gr) and lengths (Lc±1mm) were determined. After evisceration and deheading, the obtained gutted carcass, gutted and deheaded carcass, fillet with skin, and fillet without skin were weighed (±1gr), and their percentages in total body weight of fish were calculated.
2.2. Proximate Composition Analysis
- Water content was determined by the sample drying technique at a temperature of 105Ċ; crude protein content with the use of the kjeldal method with 6.25 multiplier; fat content with Blight & Dyer method; and ash content by sample mineralization at a temperature of 550-600Ċ, (Zmijewski et al, 2006).
2.3. Determination of Fatty Acid Profile
- Total lipid was extracted by chloroform-methanol and estimated (1:1, v/v) gravimetrically,(Blight and Dyer, 1959). The fatty acids in the total lipids were esterifies intomethyl esters by saponification with 0/5 N methanolic NaOH and transesterifies withBF3 (w/v) methanol (A.O.A.C.,1990) The FAMEs were analyzed on HP5890 Series II GC equipped with gas chromatograph with a FID and a 30m capillary column with an internal diameter of 0.25 mm, liquid phase Supelco wax 10, film thickness 0.25 µm. Separation conditions: nitrogen carrier gas, flow rate of 1µL/min. Temperature of the detector was 260 Ċ; of the injector250 Ċ; and of the column, 155Ċ. The individual fatty acids were identified by comparing their retention times with the standards of Supelco.
3. Statistical Analysis
- The results presented for fish of particular species are means ± S.E.M. obtained in the analysis of 8 fish. The differences between the mean values of examined parameters (biological factors, slaughter yield, proximate composition, fatty acid composition,) were calculated using Test Kolmogorov – Smirnov and Kruskal-Wallis test and analysis of variance, statically significant differences were reported at P>0.05.
4. Results
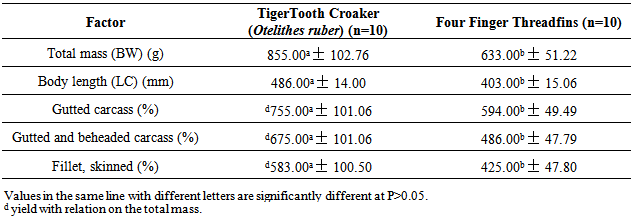
- Table 1 presents the condition factor and slaughter yield of carcasses and fillets, for Tiger Tooth Croaker and Threadfins; whose total weight accounted for 855.00±102.76g and 633.00±51.22g, respectively. The slaughter yield of the gutted carcass of experimental species did significantly differ. However, Tooth Croaker had the highest yield of gutted and de headed carcass. High yield of fillets with skin were also obtained in Tiger Tooth Croaker, totaling 583.00±100.50g
|
|
|
|
5. Discussion
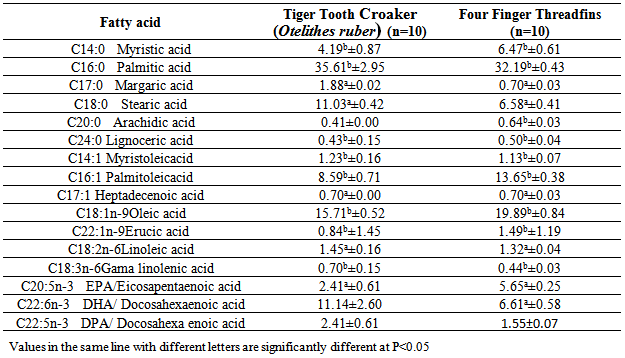
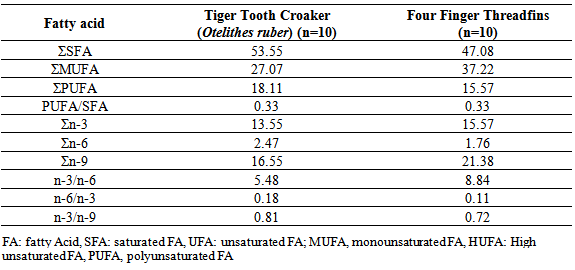
- The crude fat content of threadfins was higher than the amount found in Aspius aspius, Exoux lucius, Mugil cephalus, Alosa caspia caspia, Rutilus frisii kutum, Abramis brama, Cyprinus carpio, Oreochromis niloticus, O.macrochir, Tilapia rendalli (Zmijewski, et al., 2006, Celik, etal., 2005, Zuraini, et al., 2006). The crude fat content of threadfins was lower than Channa stiratus 5.17± 1.9%, Channa Lucius 11.9±4.3% and Channa micropeltes 9.3 ±2.7%, (Zuraini, et al., 2006).The varied content of fat was compensated by the content of water. The obtained results confirmed a reverse correlation between the fat and water contents which is common for many fish species. Fat content is influenced by species, seasons, geographical regions, age and maturity.Higher concentration of three quantitatively dominating saturated fatty acids compare to Threadfins carcass: Meristic acid (14:0), Palmitic acid (16:0) and Stearic acid (18:0). The SFA values in this study is higher than the values in Alosa caspia caspia, Chanus striata, Abramis brama, Aspius aspius,Rutlis frisii kutum, Sander luciperca, Mugil cephalus, (Sengor etal, 2003 , Zmijewski et al, 2006, Hedayati fard et al, 2008, Aliu-paik et al, 2012, jalili, 2012, jalili et al, 2013). Palmitic acid (C16:0) was the primary saturated fatty acid, contributing approximately 34.95% and 32.19% to the total saturated fatty acid content of the lipids in fresh samples of 2 examined fish. Similar results for this species (Sander luciperca), (Spratus aurita), (Boops boops); (Mugil cephalus); (Sole solea), (Paralithodes camtschaticus), (Paralithodes platypus), (Chionoecetes opilio), (Chionoecetes angulatus), (Chionoecetes japonicus), (sparus sparus), green crab (Carcinus mediterraneus) from the Tunisian Mediterranean coasts, confirm this present study. (Celic, et al, 2005, Zuraini etal, 2006, Ozogul etal, 2007, Mnari etal, 2007, Latyshev, et al, 2009, Cherif, etal, 2009, Ahmed Louly et al, 2011).The MUFA concentration of Threadfins carcass was higher that Tiger Tooth Croaker. The total sum of n-3 fatty acids reaching forTiger Tooth Croaker and Threadfins 13.55% and 15.57% respectively. Among them, those occurring in the highestTiger Tooth Croaker proportions in fresh samples were oleic acid, palmitic acid,Palmitoleic acid, DHA, EPA. These results are in agreement with previous studies on other species, (Celic et al, 2005, Ozogul et al, 2007, Mnari et al, 2007, Abourg, et al,2004) .The major fatty acids identified in Tiger Tooth Croaker and threadfins fish were C16:0, C18:0, C16:1, C18:1n-9, C18: 2n-6, C18:3n-6, C22:6n-3 (DHA), C20:5n-3(EPA), C22:5n-3 (DPA). The concentration of individual n-3 acids, including C22:6n-3 (DHA), C20:5n-3(EPA), C22:5n-3 (DPA), in the carcass of 2 examined fish. The obtained results indicate the concentration of EPA in Tiger Tooth Croakeris lower than Threadfins, In addition, Tiger Tooth Croaker has the highest concentration DHA. Among, the n-3 series, both fish are good sources of EPA (2.41%, 5.65%), DHA (11.14%, 6.61%) and EFAs help in reducing the cholesterol level and stopping blood platelets from clinging to one another, (Hedayatifard et al, 2008).Fresh water fish normally contain more n-6 PUFAs, whereas marine fish are rich in n-3 fatty acid, especially DHA and EPA, (Mnari, et al 2007, Ahmed Louly, et al, 2011). The UK department of health recommends an ideal ratio of n-6/n-3 of 4.0 at maximum. Values higher than the maximum values are harmful to health and may promote cardiovascular disease. In this study, the ratio of n-6/n-3 was found to range from 0.09 to 0.11 for Grouper and Threadfins.Fish contain certain PUFA which regulates prostaglandin synthesis and induce wound healing. The addition of n-3 PUFA could improve the nutritional value and protect against diseases. A minimum value of PUFA/SFA ratio recommended is 0.45, which is lower than those obtained from 2 studied fish species. (Ozogul, et al, 2007).Differences in fatty acids of marine and fresh water fishes should not only be considered with respect to species habitat but also based on their natural diet, especially whether a species is herbivorous, omnivorous or carnivorous, (Celick et al, 2003). It has been reported that the types and amounts of fatty acids in fish tissue vary with geographic location, size, age, reproductive status and season (Ozogul et al, 2007, Guler, et al, 2008).
6. Conclusions
- Seafood origin proteins and fatty acids play important roles in the human diet. The fatty acids PUFAs and HUFAs are crucial in terms of human feeding physiology. These fatty acids contain five or six double bonds in their body. Grouper and Threadfins live in the warm waters of Persian Gulf. They are in different ecological group, but we cannot see any differences in fatty acid profiles, but these valuable fish in Khuzestan coastal waters are very important for local consumers. Our research has shown that Threadfins is semi- fatty fish but grouper fish is a rich source of omega3. Both examined fish are highly recommended for children’s growth, pregnant woman and fetal health.
ACKNOWLEDGEMENTS
- This paper has been prepared by research project, Department fisheries, Islamic Azad University, Abadan Branch.
Notes
- 1. Tri Fluoride Bore2. Fatty Acids Methyl Esters3. Gas Chromatograph4. Flame Ionizing Detector
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML