Retno Suntari1, 2, Rurini Retnowati3, Soemarno2, Mochammad Munir2
1Agricultural Sciences Graduate Program, Faculty of Agriculture, University of Brawijaya, Indonesia
2Soil Science Department, Faculty of Agriculture, University of Brawijaya, Indonesia
3Chemistry Department, Faculty of Mathematics and Natural Sciences, University of Brawijaya, Indonesia
Correspondence to: Retno Suntari, Agricultural Sciences Graduate Program, Faculty of Agriculture, University of Brawijaya, Indonesia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
N dynamics in the soil can be determined from the information about the release of N from N fertilizer applied to the soil until it becomes N-available to plants. Urea-humate dynamics in ricefield is not yet widely known. Application of urea-humate in flooded soil is believed to be able to increase N in the form of N-NH4+ and increase soil pH. The purpose of this study was to examine the effects of flooding (water saturated and field capacity soil conditions) on the dynamics of urea-humate. Four treatments (field capacity urea, field capacity urea-humate, flooded urea, and flooded urea-humate) are used as incubation treatments on ricefield Vertisol being studied. Results of experiment in the greenhouse showed that flooding with depth of water 5 cm from the soil surface and treatment of urea-humate 260 ppm can increase the soil pH and reduce soil Eh. Urea-humate in flooded soil until week 5 still produces N-NH4+ that is higher than urea, and N-NO3- levels were higher in conditions of field capacity. Furthermore, pH, Eh, and soil temperature are factors that affect the availability of N-NH4+ and N-NO3-.
Keywords:
Urea-humate, N-NH4+, N-NO3-, Flooding, pH, Eh
Cite this paper: Retno Suntari, Rurini Retnowati, Soemarno, Mochammad Munir, The Effect of Flooding and Application of Different Urea on Soil Chemical Properties and N-Available (NH4+ and NO3-) on Vertisols, International Journal of Ecosystem, Vol. 3 No. 6, 2013, pp. 196-202. doi: 10.5923/j.ije.20130306.05.
1. Introduction
One of the reasons behind land’s low productivity is the low efficiency of nitrogen fertilizer applied by farmers, various soil factors can cause the low availability of N from fertilizer. Factors affecting the release of N-available from urea-humate fertilizer are: humic acid concentration, soil pH, Eh, temperature and CEC[4,8,12,23]. Flooding causes soil pH near neutral, it encourages mineralization of N-organic into N-NH4+ which is available for plants. In flooded conditions, soil Eh is low and can be attributed to the increasing accumulation of N-NH4+. In contrast, flooded soils with increased pH value may trigger changes of N-NH4+ to NH3 which can evaporate. In these conditions (low Eh, high pH), nitrogen can also be released in the form of N2 and N2O as a result of the denitrification process. Low levels of soil fertility and productivity is a major obstacle in ricefield management[10]. Vertisol has high CEC, high base saturation, and very slow permeability, which makes it suitable for ricefield. Vertisol has a smooth texture with clay mineral content type 2:1, so it becomes very hard when dry and becomes sticky when wet. Additionally, Vertisols have relatively high pH, unbalanced nutrient status, and low organic matter content (<1%) so its N content is also low (0,08-0,18%)[18]. Nursyamsi et al.[15] reported that in flooded soil can occur chemical and electronics changes that are detrimental to the growth of plants. These changes include: (1) decrease of redox potential, (2) lack of oxygen, and (3) reduction of Fe3+ to Fe2+, Mn4+ to Mn2+, NO3- and NO2- to N2O and N2. Min et al.[13] showed that in the flooded state, the loss of NO3- to the soil layers is very rapid. Transport of NO3- is influenced by the clay content. Reduction of soil does not inhibit the growth of rice plants except at higher Eh value of -300 mV, where sulfide can be produced at a rate that poisoned[17]. Reduction processes cause a decrease in Eh and produce OH- ions thus increasing the pH[22]. The higher the organic matter content of the soil, the greater the reduction power. Effects of flooding are increasing the pH of acid soil and decrease the pH of alkaline soils. Changes in the pH value is due to the release of OH- ions of Fe (OH)3 to Fe (OH)2. Alkaline pH values in the soil was reduced to 7 due to the increase in CO2 partial-pressure that produces H+ ions[1,7,17,22]. Soils with good aeration usually have redox potential values between +400 - 700 mV [7]. Fausey and Lal[6] reported that the flooded soil can have a redox potential of -300 mV. Under the Eh value of +400 mV, the soil is classified in the "moderate-reduction", while at -100 mV is classified in the "high-reduction". The value of Eh affects the availability of the elements for plant. There have been studies about the absorption of 5 macro elements and 10 micro elements by maize in flooded soil compared to well drained soil using lysimeter. The result shows that the absorption of Al, Fe, Mn, and Mo increased but the absorption of N, P, K, Zn, Cu, and B decreased in flooded conditions. Panda[16] reported that the amount of NH4+ release from ricefield (4-11%) experienced an increase in flooding with water depths up to 10 cm. Furthermore NH4+ content decreased at a water depth of 30 cm. The high amount of NH4+ in the soil with the flooding of up to 5 cm compared with soil that is not flooded is due to the large number of ammonification bacteria and the small number of nitrosomonas and nitrobacter bacteria on flooded soils or the inactive aerobic bacteria. Anilakumar et al.[3] also found that the addition of N fertilizer from several N sources resulted in waterlogged soil Eh was lower than the control treatment without N fertilizer. Compounds most likely to be involved in buffering the pH of flooded soil are Fe and Mn compounds in the form of hydroxide carbonate and carbonic acid. This flooding may lead to the increased availability of nutrients[22]. Panda[16] suggests that the flooding increases production of NH4+ in the soil, organic N mineralization in flooded soil is faster than in non-flooded soil.
2. Materials and Methods
The research was conducted from October 2011 to November 2011 in the laboratory and greenhouse of University of Brawijaya, Indonesia. Soil samples at a depth of 0-30 cm were collected from The Experimental Station of Research Center for Legume and Tuber Crop, Ngale, Ngawi, East Java, Indonesia. Soil samples used has been refined through 20 mesh sieve.Urea-humate 260 ppm used is an engineered mix between urea and potassium humate formula (KH 26) obtained from Leonardite sediment of Victoria's gippland Australia. Manufacture of urea-humate 260 ppm is by mixing 100 mL KH 26 containing 26% humic acid in 1 kg of urea. Urea used in this research is urea 46% N, a product of Pupuk Kalimantan Timur, Bontang, Indonesia. Concentration of humic acid used is based on research results from Khaled and Fawy[11], where the addition of humic acid through the soil is more economical if provided at a concentration of <0,2% and provision through the leaves at a concentration of <0,1%.1 kg of air-dry soil which has been refined through sieve is included in volume 3 L plastic pots and then watered and stirred up until it silt. For flooded treatment, water in plastic pots maintained at a height of 5 cm. Incubation treatment consists of 2 factors, namely the type of fertilizer and flooding. The first factor is the type of fertilizer: urea 46% (U) and urea-humate 260 ppm (UH). The second factor (T) is a continuous flooding (+ 5 cm) for 5 weeks and the condition of field capacity (KL). The amount of fertilizer applied per pot was 0.1 g kg-1 in accordance with the recommended dose of urea fertilizer for rice in Ngale, Ngawi i.e. 200 kg ha-1.Observations were made every week for 5 weeks (5 times of observation). Soil and water sampling were done for analysis of ammonium and nitrate method given by Anderson and Ingram[2]. Analysis of chemical and physical properties of soil using standard procedures. Analysis of C-organic using Walkey and Black method[19]. Analysis of N-total, N-NH4+ and N- NO3- using Kjeldahl, CEC with NH4OAc 1N pH 7[9]. pH and Eh were measured with Multi Water Quality Checker V-50.Then tested statistically using factorial CRD with F test level of 5%. Followed by Duncan's test at the 5% level to see the effect differences between treatments. Multiple regression test is done to see the interrelation of pH, Eh, CEC and temperature on the availability of N-NH4+ and N- NO3-.
3. Result and Discussion
3.1. pH (H2O) in the Soil
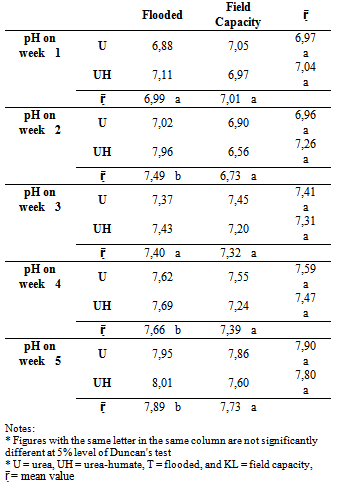
The addition of urea-humate 260 ppm both on the state of field capacity (KL) and the flooded state (T), the pH of the soil during the five weeks of incubation was higher than the initial pH value of 6,30.Table 1. The effect of flooding on soil pH
 |
| |
|
At week 1 of flooding, soil pH increased to 6,88 on urea treatment and to pH 7,11 on urea-humate treatment. While on field capacity treatment, soil pH value increased to 7,05 (urea) and 6,97 (urea-humate) (Figure 1). Both showed that at week 1 soil pH changed from slightly acidic to neutral conditions. This is contrast to urea-humate 260 ppm treatment which increased sharply in week 2, in flooded state the soil pH changed from 7,11 to 7,96 (slightly alkaline), while in urea treatment from 6,88 to 7,02 (Table 1).Sanchez[22] showed that one month after flooding nearly all types of soil to reach pH 6,6 – 7,2 and those value are fixed until the soil is dry.Table 1 shows that the effect of flooding is very significant and there is interaction between flooding and urea on the soil pH in incubation at week 1 to week 5. At week 2, the soil pH at field capacity condition declined from 7,01 to 6,73 and at flooded condition increased from 6,99 to 7,49 but still within the range of neutral. This is in accordance with the opinion of Purakayastha et al.[20] that the Vertisol pH declined sharply in week 2. Table 1 shows that flooding significantly gives a higher pH than field capacity on incubation weeks 2, 4, and 5.Sanchez[22] adds that the reduction process produces OH- ions so as to increase the soil pH. The higher the organic matter content of the soil, the higher the reduction power. Increase in the pH value is also due to the release of OH- from Fe(OH)3 into Fe(OH)2 given urea-humate 260 ppm used also contains Fe.Increase in pH up to five weeks of flooding in the treatment of urea-humate reached 8,01 and 7,95 (urea) while in a state of field capacity reached pH 7,86 (urea) and 7,6 (urea-humate). It is also influenced by the results of analysis which shows that the humic acid used containing carboxyl group (71,4 cmol kg-1) and OH-phenol group (101,7 cmol kg-1) which can form a chelate with metal elements[24].Humic acid is a polyelectrolyte macromolecules capable to chelate metal ions[24]. This causes a decrease in the solubility of the metal ions so as to increase the soil pH. Wang et al.[25] also showed that at neutral to alkaline pH, humic acid functional groups can undergo deprotonation resulting in lower hydrogen bonding and increase the amount of negative charge. Saidi[21] adds that the pH value greatly influenced by the type of soil and the addition of organic material after 5 weeks of flooding.
3.2. Soil Eh
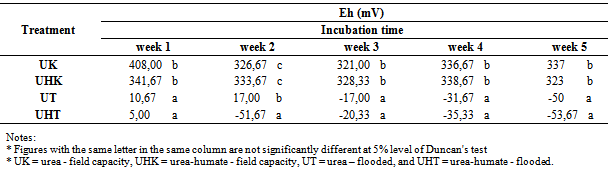
Soil Eh on incubation at week 1 to week 5 are presented in Table 2 and Figure 2.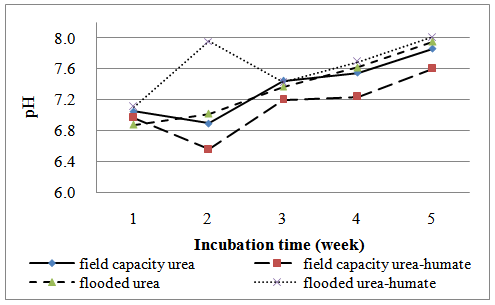 | Figure 1. The effect of flooded on soil pH |
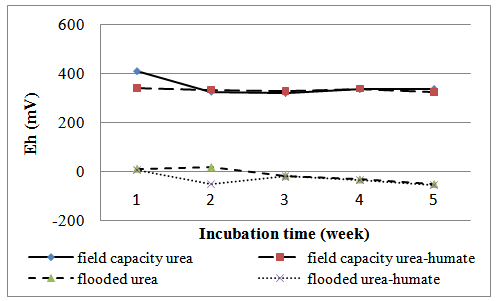 | Figure 2. The effect of flooding on soil Eh |
Table 2. The effect of flooding on soil Eh
 |
| |
|
Flooding can decrease soil Eh, soil Eh both on urea-humate and urea are lower than the Eh in the state of field capacity (Table 2). Statistical analysis showed that the Eh value very significantly affected by the flooding treatment. Eh value in the state of field capacity on incubation for 5 weeks ranged between 321-408 mV (urea) and 323 to 341,67 mV (urea-humate). Differ highly significant with a value of Eh in flooded conditions in the range of -50 mV to 17 mV (urea) and -53,67 mV to 5 mV (urea-humate). This is shown in incubation at week 1, which urea-humate Eh reached 341,67 mV (K) and 5 mV (T). Furthermore, at week 2 urea-humate Eh values decreased to 333,67 mV (K) and -51.67 mV (T). At week 3 to 5, Eh of flooded soils tends to decrease, both the urea and urea-humate. This is in accordance with the opinion of Sanchez[22] that the flooding resulted in decreased soil redox potential (Eh). Eh decreased sharply and reached a minimum in several days then rose rapidly to reach a maximum value and then decreases again.At field capacity conditions, Eh urea treatment at week 3 tends to increase until week 5. While urea-humate treatment tends to increase until week 4 but decreased at week 5. This is in accordance with the opinion of Saidi[21] that the addition of organic matter can lower soil Eh value from 278,3 mV to -213 mV at week 5 after flooding Vertisol soil. According to Sanchez[22], reduction processes cause a decrease in soil Eh.
3.3. Soil CEC
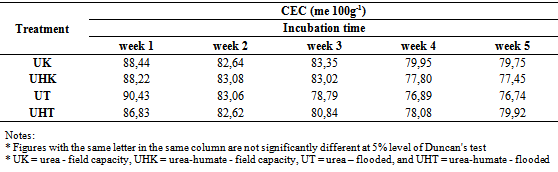
Soil CEC is an important criterion of soil fertility which can reflect the ability to provide and keep nutrients in the soil. This is related to the use of fertilizers, where cation which are fixed to the soil with high CEC can be used as a backup nutrients for plants.Soil CEC at field capacity and flooded conditions, both in the application of urea and urea-humate included in the very high criteria, ie 76,74 to 90,43 me 100g-1 (flooded) and the condition of field capacity from 77,45 to 88,44 me 100g-1. CEC on flooded and field capacity decreased until week 4 and subsequently stabilized through week 5, except for the treatment of urea-humate under flooded conditions that increased again at week 5. Statistical test results showed no significant differences between treatments (Table 3). The high humification of soil organic matter with high cation exchange capacity (CEC) binding most N-NH4+ on the exchange complex. Further N-NH4+ experienced less nitrification[27]. On the other hand, the high CEC of Vertisol also influential in reducing the loss of NH3 in the soil[20].Table 3. The effect of flooding on soil CEC
 |
| |
|
The addition of urea-humate at 260 ppm concentration does not affect the value of Vertisol’s CEC. Thus, the addition of urea-humate does not increase the availability of N-NH4+. This is consistent with the results of research from Narteh and Sahrawat[14] which indicates that the soil CEC and clay content of the soil is have no significant correlation with the content of N-NH4+.
3.4. Levels of N-NH4+ and N-NO3- of the Soil
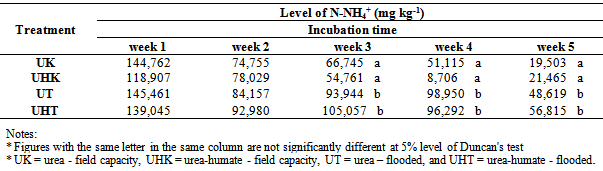
Table 4. The effect of flooding on the availability of N-NH4+
 |
| |
|
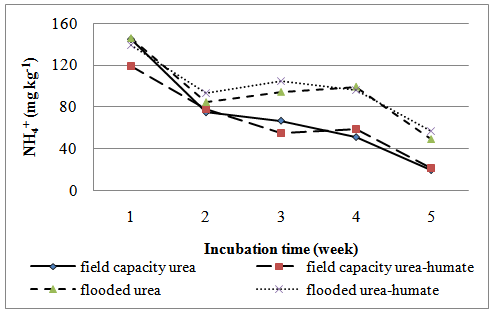 | Figure 3. The effect of flooding on N-NH4+ |
Table 5. Effect of flooding on the availability of N- NO3-
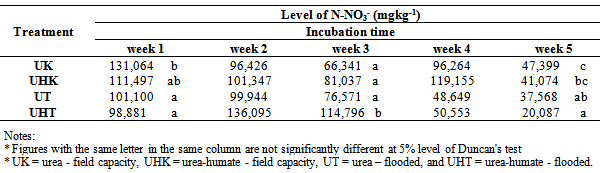 |
| |
|
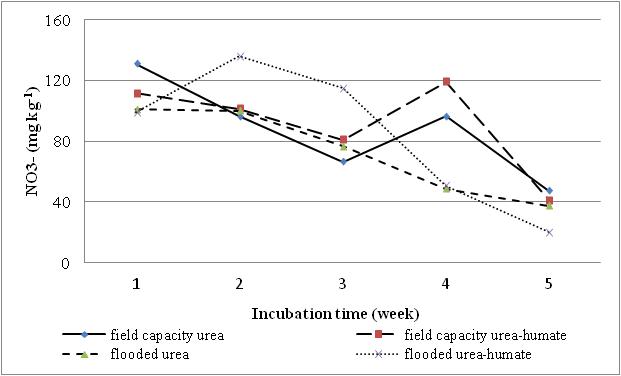 | Figure 4. The effect of flooding on N-NO3- |
Table 6. The effect of flooding on soil temperature
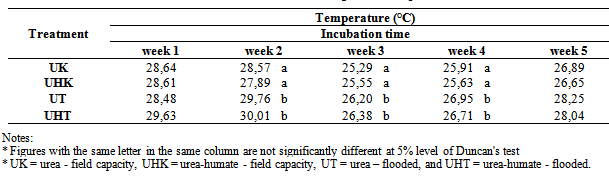 |
| |
|
Effect of flooding showed that the solubility of N-NH4+ in the treatment of urea and urea-humate decreased at week 2 and increased again at week 3, then decreased until the 5th week of incubation. Levels of N-NH4+ in the first week of treatment urea 145.461 mg kg-1 decreased to 48.619 mg kg-1 at week 5. While urea-humate in the first week 139.045 mg kg-1 decreased to 56.815 mg kg-1 at week 5. This suggests that the flooding could lead to the availability of most nutrients[22]. The statistical analysis in Table 4 shows that during week 3, 4, and 5 of incubation, N-NH4+ in flooded soil treatment differ highly significant with the field capacity treatment.Higher N-NH4+ in flooding up to 5 cm compared to field capacity condition may be caused by the high number of ammonification bacteria and the low number of nitrosomonas and nitrobacter bacteria on flooded soils[16].Flooding at week 1, 3, and 5 significantly affect the levels of N-NO3- (Table 5). Decreased availability of N-NH4+ is associated with the higher availability of N-NO3- in week 1 and week 5 on the condition of field capacity, which is significantly different from flooded conditions due to the process of nitrification. Until week 3, the levels of N-NO3- on urea-humate treatment on flooded soil are higher than urea treatment. This is presumably because the pH on urea-humate treatment for flooded soil also rose sharply in week 2 reaching a pH value of 7.96. According Cyio[5] this happens because there are contributions from organic materials containing Fe that undergo changes from ferric into ferrous and OH- release from nitrate to nitrite by the following reaction:Fe(OH)3 + e à Fe(OH)2 + OH-NO3- + H2O + 4e à NO2- + 2OH-Furthermore, N-NO3- decreased until the 5th week of flooding in accordance with increasing pH value. This is in accordance with opinions from Havlin et al.[8] that pH more than 7 spur N losses in the form of elements (N2). When the soil flooded, it becomes anaerobic and some organisms obtain O2 from NO2- and NO3- by releasing N2 and N2O.Denitrification reaction that occurs is: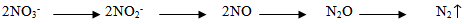 C available are needed by microbial to change 2NO3- to N2 or N2O. Denitrification will be higher when the temperature rose from 25°C to 60°C. Given the maximum temperature of the soil at the time of the study reaching 30°C (Table 6) which indicates that incubation weeks 2, 3, and 4 were significantly affected by the flooding treatment. On the other hand, the value of N-NH4+ from urea-humate in week 5 is still higher than the urea treatment both at the flooded and field capacity condition. This suggests that the urea-humate fertilizer is a slow release fertilizer.
C available are needed by microbial to change 2NO3- to N2 or N2O. Denitrification will be higher when the temperature rose from 25°C to 60°C. Given the maximum temperature of the soil at the time of the study reaching 30°C (Table 6) which indicates that incubation weeks 2, 3, and 4 were significantly affected by the flooding treatment. On the other hand, the value of N-NH4+ from urea-humate in week 5 is still higher than the urea treatment both at the flooded and field capacity condition. This suggests that the urea-humate fertilizer is a slow release fertilizer.
3.5. The Relationship between pH, Eh, CEC, and Temperature to the Availability of N-NH4+ and N-NO3-
Results of analysis of variance of multiple linear regression equation is used to determine the effect of several parameters (pH, Eh, CEC, and temperature) on the availability of N-NH4+ and N-NO3-. Equation for N-NH4+ is as followsy = 26,128 x1 - 0,058 x2 - 0,680 x3 + 1,825 x4 - 95,119 (R2 = 0,964)where y = N-NH4+; x1 = pH; x2 = Eh; x3 = CEC, x4 = temperatureThis equation shows that the availability of N-NH4+ is very significantly affected by pH, Eh, and temperature. This is supported by calculated F value of 47.386 with a significance level of 0.000 and R2 = 0.964 which indicates that 96.4% availability of N-NH4+ is influenced by parameters observed. This is in accordance with the opinion of Havlin et al.[8] that N mineralization depends on temperature, pH, and soil water content.While the results of the analysis of variance of multiple linear regression equation for N- NO3- are as follows:y = 1,542 x1 + 0,061 x2 - 2,193 x3 + 1,055 x4 + 210,743 (R2 = 0,875)where y = N- NO3-; x1 = pH; x2 = Eh; x3 = CEC, x4 = temperatureThis equation shows that the availability of N-NO3- is very significantly affected by soil Eh. This is supported by calculated F value of 12.291 with a significance level of 0.003 and R2 = 0.875 which indicates that 87.5% availability of N-NO3- is influenced by the observed parameters (pH, Eh, CEC, and temperature). This is in accordance with the opinion of Sanchez[22] which states that nitrate becomes unstable at the Eh value of +400 mV and +300 mV due to denitrification.
4. Conclusions
Based on the results and discussion that has been done, it can be concluded that:1. The urea-humate 260 ppm in flooded conditions may increase soil pH and decrease soil Eh value,2. The urea-humate in flooded soil, until week 5, still produces higher N-NH4+ than urea, but the N- NO3- levels were higher only until week 3, and3. pH, Eh, and temperature of the soil are factors that affects the availability of N-NH4+ and N- NO3-.
ACKNOWLEDGMENTS
This research was funded by the Directorate General of Higher Education of the Ministry of Education and Culture, Indonesia through the Agricultural Sciences Graduate Program, Faculty of Agriculture, University of Brawijaya, and supported by facilities from Soil Chemistry Laboratory, Faculty of Agriculture, University of Brawijaya, Malang-Indonesia.
References
| [1] | Ahn, P.M. 1993. Tropical Soils and Fertilizers Use. Longman Group. UK. Limited England. p 264. |
| [2] | Anderson, J.M, and J.S.I. Ingram. 1989. Tropical Soil Biologi and Fertility. A Handbook of Methods. CAB. International. pp 82-85. |
| [3] | Anilakumar, K., K.P. Rajaram, and C. Sivakumar. 1994. Effect of nitrogen on chemical properties of flood water. J. Indian Soc. Soil Sci. 42 (4): 647-649. |
| [4] | Breuer, L., R. Kiese, and K. Butterbach-Bahl. 2002. Temperature and Moisture Effects on Nitrification Rates in Tropical Rain-Forest Soils. Soil Sci. Soc. Am. J. 66: 834-844. |
| [5] | Cyio, M.B. 2008. Efektivitas Bahan Organik dan Tinggi Genangan Terhadap Perubahan Eh, pH, dan Status Fe, P, Al Terlarut pada Ultisol. J. Agroland 15 (4): 257-263. |
| [6] | Fausey, N.R. and R. Lal. 1990. Soil Wetness and Anaerobiosis. In Advances in Soil Science Volume 11. Springer-Verley. New York Inc.: 173-185. |
| [7] | Hardjowigeno, S. dan M.L. Rayes. 2005. Tanah Sawah. Bayu Media Publishing. Malang. p 208 |
| [8] | Havlin, J.L., J.D. Beaton, S.L. Tisdale and W.L. Nelson. 1999. Soil Fertility and Fertilizer: An Introduction to Nutrient Management. Prentice Hall Inc.Upper Saddle River. New Jersey. pp 86-153 |
| [9] | Hidayat, A. 1978. Methods of Soil Chemical Analysis. JICA. Central Research Institut for Agricultural. Bogor. Indonesia. pp 30-39 |
| [10] | Kasijadi, F., Suyanto dan M. Sugiyanto. 2000. Rakitan Teknologi Budidaya Padi, Jagung, dan Kedelai. BPTP Karangploso. Malang. p 44 |
| [11] | Khaled, H. and H.A. Fawy. 2011. Effect of Different Levels of Humic Acids on the Nutrient Content, Plant Growth, and Soil Properties under Conditions of Salinity. Soil and Water Res. 6 (1): 21-29. |
| [12] | Kyveryga, P.M., A.M. Blackmer, J.W. Ellsworth, and R. Isla. 2004. Soil pH Effects on Nitrification of Fall – Applied Anhydrous Ammonia. Soil. Sci. Soc. Am. J. 68: 545-551. |
| [13] | Min, C.X., W.H. Shan and W. Fei. 2007. Nitrat Vertical Transport in the main paddy soil of Tai Lake Region China. Geoderma.142(1-2) :136-137. |
| [14] | Narteh, LT. and K.L. Sahrawat. 2000. Ammonium in solution of flooded west African Soils. Geoderma. 95 :205-214. |
| [15] | Nursyamsi, D., L.R Widowati, D.Setyorini dan J. Sri Adiningsih. 2000. Pengaruh Pengolahan Tanah, Pengairan Terputus dan Pemupukan Terhadap Produktivitas Lahan Sawah baru pada Inceptisol dan Ultisol Muarabetiri dan Tatakarya. Jurnal Tanah dan Iklim 18 :29-38.. |
| [16] | Panda, M.M. 1994. Influence of depth of standing water on net ammonium release from flooded rice field soil. J. Indian Soc Soil Sci. 42 (2): 324-325. |
| [17] | Prasetyo, B.H., J. Sri Adiningsih, K. Subagyono, dan R.D.M. Simanungkalit. 2004. Mineralogi Kimia, Fisika, dan Biologi Tanah Sawah. dalam Tanah Sawah dan Teknologi Pengelolaannya. BPPT dan Agroklimat. Bogor. pp 29-82 |
| [18] | Prasetyo, B.H. 2007. Perbedaan Sifat-Sifat Tanah Vertisol Berbagai Bahan Induk. Jurnal Ilmu-Ilmu Petanian Indonesia. 9: 20-31. |
| [19] | Prawirowardoyo, S.A.. Roesmarkam, D. Shiddiq, S. Hidayat dan M. Mashum. 1982. Prosedur Analisa Kimia Tanah. Dep. Ilmu Tanah. FP. UGM Yogyakarta. p 43. |
| [20] | Purakayastha, T.J, J.C. Katyal and N.N. Goswami. 1997. Evaluation of ammonia volatilization from some modified urea fertilizer. J. Indian Soc Soil Sci. 45 (1): 9-14. |
| [21] | Saidi, D. 1999. The Effect of Organic Matter on pH, Eh, and Fe Change in Submerged Ultisol and Verstisol. Agrivet. Majalah Ilmiah Fakultas Pertanian UPN Veteran Yogyakarta.3 (1): 8-20. |
| [22] | Sanchez. 1976. Properties and management of Soils in The Tropics. John Wiley and Sons. New York. p 618. |
| [23] | Sarir M.S., M.I. Durrani dan I.A. Mian. 2006. Effect of the source and rate of humic acid on phosphorus transformations. Journal of Agricultural and Biological Science 1 (1): 29-31. |
| [24] | Tan, K.H. 1991. Principle of Soil Chemistry (Dasar-dasar Kimia Tanah)(. Alih bahasa: Didiek Hadjar Goenadi) Gadjah Mada University Press. Yogyakarta. p 295 |
| [25] | Wang, Y., C. Combe, and M.M. Clark. 2001. The effects of pH and calcium on the diffusion coefficient of humic acid. Journal of Membran Science. 183: 49-60. |
| [26] | Wild, A. 1993. Soil and the Environment: An Introduction. Cambridge University Press. p 287. |
| [27] | Xie R.J., A.F. Mac Kenzie, L.P.O. Hallovan and J.W. Fyles. 1994. Concurrent transformation of Lignosulfonate carbon and Urea N in clay soil. Soil Sci Soc Am J. 58 :824-828. |




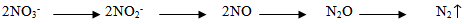 C available are needed by microbial to change 2NO3- to N2 or N2O. Denitrification will be higher when the temperature rose from 25°C to 60°C. Given the maximum temperature of the soil at the time of the study reaching 30°C (Table 6) which indicates that incubation weeks 2, 3, and 4 were significantly affected by the flooding treatment. On the other hand, the value of N-NH4+ from urea-humate in week 5 is still higher than the urea treatment both at the flooded and field capacity condition. This suggests that the urea-humate fertilizer is a slow release fertilizer.
C available are needed by microbial to change 2NO3- to N2 or N2O. Denitrification will be higher when the temperature rose from 25°C to 60°C. Given the maximum temperature of the soil at the time of the study reaching 30°C (Table 6) which indicates that incubation weeks 2, 3, and 4 were significantly affected by the flooding treatment. On the other hand, the value of N-NH4+ from urea-humate in week 5 is still higher than the urea treatment both at the flooded and field capacity condition. This suggests that the urea-humate fertilizer is a slow release fertilizer. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




