-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2013; 3(5): 140-147
doi:10.5923/j.ije.20130305.06
Effects of Slash-and-Burn Farming System on Arthropods’ Populations Density and Soil Physical Conditions in Acid Sands
Edem Dennis 1, Mfon Ekanem 2, Rosemary Essien 3
1Department of Soil and Land Resources Management, Faculty of Agriculture, University of Uyo, Nigeria
2Department of Zoology, Faculty of Science, University of Uyo, Nigeria
3Department of Crop Science, Akwa Ibom State University, Obio Akpa, Nigeria
Correspondence to: Edem Dennis , Department of Soil and Land Resources Management, Faculty of Agriculture, University of Uyo, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Arthropods’ populations tend to increase with practices the increases soil organic matter level and minimal disturbances. Soil fauna work as engineers, initiating the breaking down of dead plant and animal material, ingesting and processing large amount of soil, burrowing biopore for water and air movement, mixing soil layers and increasing aggregation. This natural capital is stressed by slash-and-burn farming system to the point where the natural feedback loops to sustain a balance in the soil ecosystem and soil life is impaired. Between 2010 and 2011, an experiment was conducted in the humid tropical soil of Uyo, Nigeria, to examine the effects of vegetation burning affects on the population density of arthropods, and how key soil physical conditions related to one another changed during farming activities. The selected area of 8% slope was divided into three (3) main plots (3 m wide and 10 m long) of which three sub-plots each measuring 3 x 3 m2 with a fireproof tract (0.3 m) except the control were imposed with three different levels of dry biomass (100, 150 and 200 kg per square meter) that produces different heating intensities in a randomized complete block design arrangement (RCBD). The results showed that between 5 and 10 cm depths, more than 88% of the arthropods were either destroyed by excessive heat or burrowed beyond these depths. Heat resulting from burning 100, 150 and 200 Kg DM m-2 affected the arthropods in the order of 60, 98, and 70% at 5 cm depth, whereas at 10 cm depth, the effects were 45, 60 and < 1 % respectively across the field. The relatively rapid increase in Ksat with high bulk density in the 150 and 200 Kg Dm m-2 in the burnt plots were attributed to the increased presence of Archoplophora rostralis that rebound after burning. Therefore, arthropod burrows reportedly make up a small portion of the total soil volume, and their relatively large size and connectivity make them important conduits for water movement.
Keywords: Burning, Fauna, Arthropods, Population, Temperature, Soil Properties
Cite this paper: Edem Dennis , Mfon Ekanem , Rosemary Essien , Effects of Slash-and-Burn Farming System on Arthropods’ Populations Density and Soil Physical Conditions in Acid Sands, International Journal of Ecosystem, Vol. 3 No. 5, 2013, pp. 140-147. doi: 10.5923/j.ije.20130305.06.
Article Outline
1. Introduction
- Some soil cultivation is often reported to be detrimental to soil fauna especially when the method of land clearing is slash and burn. Farmers are becoming increasingly unaware that the capital value in a soil is primarily the abundance and diversity of arthropods, microbes, soil carbon, organic compounds and soil physical attributes[1]. The practice of burning is not a new idea but started many generations ago with the burning of grass lands. Burning is an inexpensive, labour efficient means of removing unwanted crop residues prior to tillage or seedbed preparation[2]. If completed correctly, burning can be an effective management tool. Litton et al.,[3] reported that with the increased use of reduced tillage equipment and tillage methods, farmers and rangers have reduced the acres they annually burn to eliminate excess residue and vegetation. It is important to understand how plants utilize nutrients from soil and how burning affects available soil nutrients. Long-term annual burning results in lower levels of soil organic matter and net N mineralization rates[4], but higher levels of plant productivity is obtained, compared with no burning. Burning also results in changes in soil temperature, soil moisture and fauna and nutrients availability[5]. Burning grasslands results in short term increases in nitrogen mineralization which results in short burst of nutrient available for the plant. Burning frequently has detrimental effects. Some of these effects are: removal of the extra vegetative material that would add humus and N to the soil, destruction of old vegetation in the soil which functions to increase water-holding capacity, and injury to living arthropods, shallow-rooted vegetations which may be killed in a single burning. The extent of fire effects on soil properties varies considerably depending on fire intensity, fire severity and fire frequency. According to NWCG[6], most fires do not cause enough soil heating to produce significant changes to soil physical properties, but intense burning may have detrimental effect on soil properties by consuming soil organic matter and fauna. Soil organic matter holds sand, silt and clay particles into aggregate. Therefore, a loss of soil organic matter results in a loss of structure. By altering soil structure severe fires can increase soil bulk density[7] and reduce soil porosity, mostly through the loss of macro pores (> 0.6mm diameter). Soil porosity can also be reduced by the loss of soil invertebrates that channel in the soil. There is no doubt that fire has a dramatic influence on the soil macro and micro nutrients. Fires whether planned or not, usually decrease the total site nutrient pool through some combination of oxidation, volatilization, ash transport, leaching and erosion. Recent researches has shown that, although there are some short-term benefits to burning crop residues and grassland, there are long-term detrimental effects to soil quality, fauna and overall cropland production. This fact sheet summarizes the effects of slash-and-burn agriculture on soil nutrient pools and biological spectra in relation to effect of soil quality at varying burning temperatures.
 | Figure 1. akwa ibom state showing the project area |
2. Materials and Methods
2.1. Study Site
- The experiment was conducted at the University of Uyo Teaching and Research farm (UUTRF), Use-Offot in the humid tropical zone of Nigeria. The area lies between latitudes 4° 52’ and 5° 3’N and longitudes 7° 51’ and 8° 20’ E (Fig. 1) and altitude 65 m from the sea level. The area has two distinct seasons, the wet and dry seasons. The wet or rainy season begins from April and lasts till October. It is characterized by heavy rainfall of about 2500-4000 mm per annum. The rainfall intensity is very high and there is evidence of high leaching and erosion associated with slope and rainfall factors in the area[8]. The dry season lasts from November to March. Temperature is moderately high varying from 26℃ to 30℃ throughout the year, and high relative humidity of 70 and 80%. The highest temperature is often experienced between January through March, the period described by Udo-Inyang and Edem[9] as overhead passage of the sun. The landscape is generally undulating to steep hills while the vegetation is mainly, the secondary rain forest type. The soils are derived from sandy parent materials which are weathered with low activity clay. The predominant land use is continuous cropping. Before the experiment, the prevalent vegetation in the area was Panicum maximum, Aspilia africana and Imperial cylindrical.
2.2. Layout of Experimental Plots
- Before the layout of experimental plots, the land was manually cleared the trashes were left on the ground surface for a week to dry. The selected area was divided into three (3) main plots (3 m wide and 10 m long) of which three sub-plots each measuring 3 x 3 m2 with a fireproof tract (0.3 m) except the control were imposed with three different levels of dry biomass (100, 150 and 200 kg per square meter) that produces different heating intensities in a randomized complete block design arrangement (RCBD). Slopes ranged from 6 to 10% with an average slope of 8%. The experimental fire with the measured dry biomass was performed. The atmospheric pressure and air temperature were taken and the relative humidity determined when the winds were calmed. The maximum temperatures reached during the fires were recorded by means of soil temperature sensor
2.3. Soil Sampling and Analytical Procedures
- A total of 40 Bulk, core and aggregate samples were collected at two depths of 5cm interval (0-5 and 5-30cm). The core samples were obtained for saturated hydraulic conductivity and bulk density determinations. The soil samples were secured in a core and one end of the core was covered with a piece of calico cloth fastened with a rubber band and labeled while the bulk samples were collected and secured in labeled polythene bags before conveyance to the University of Uyo Soil Science laboratory for physical, chemical and structural parameters determinations. Particle-size distribution was determined by the hydrometer method[10]. Chemical analyses were carried out on soil samples with a particle size < 2.0 mm using standard methods and procedures as outlined by Gee and Or,[11]. Soil samples were analysed for: organic C using the Walkley and Black[12] method; total N by Kjeldahl digestion method; Water stable aggregates (WSA) were determined using modified Hubbert[13] wet sieving methods described by Nimmo and Perkins[14]. 100g of moist soil sample at 0- 15cm and 15-30cm depths were placed on a nest of sieves (2, 1, 0.5 and 0.25mm). The nests of sieves were cycled through a column of water for 10 minutes (10 cycles per mm). The percentage of water stable aggregate (WSA) fraction of the total sample was calculated. Mean weight diameter (MWD), a statistical index of aggregation was calculated from aggregate size distribution data after correction had been made for sand fractions by dispersion with sodium hexametaphosphate
2.4. Isolation of Arthropods
- Four soil cores each of 5 and 10cm depths by 5 cm diameter respectively were taken around the three replicated plots for the pre and post burnt soils. Each of the four cores per depth was collated together in a labeled calico bag and transported in a cooler with ice packs to the University of Uyo Zoological laboratory for soil arthropods extraction. Extraction of soil arthropods from the soil took place immediately, the same day samples were collected. Each sample was gently mixed together, and the clumps separated.100 grams of the collated soil samples were measured into a muslin cloth and placed in the funnel of a berlesse tullgren apparatus. Heat was applied to the soil samples from a 40 watt light bulb, and the soil arthropods were collected into 70% ethyl alcohol. The extraction lasted for 48 hours. Soil arthropods extracted were examined and identified under a dissecting microscope (Comecta Trinocular Stereomicroscope e Model SQF-E). Post burnt soil samples were collected two weeks after vegetation burning from the nine plots earlier sampled and the same procedures were followed as in pre-burned.
2.5. Statistical Analysis
- Arthropod populations were transformed with a square root function to achieve a normal distribution prior to analysis of variance. Homogeneity of variance of the transformed data was confirmed with a Bonferroni test using the PROC UNIVERATE function of SPSS statistical software (Version 17). Data from all the treatments were analysed as a randomized complete block, and error associated with the experimental blocks was included in the statistical model. The effects of burning on soil physical conditions at different burning intensities were evaluated by analysis of variance using the GLM function of SPSS software. When treatment effects were significant, mean value were compared with a post hoc Tukey-Kramer test (p <0.05)
3. Results and Discussion
3.1. Soil Physical Properties
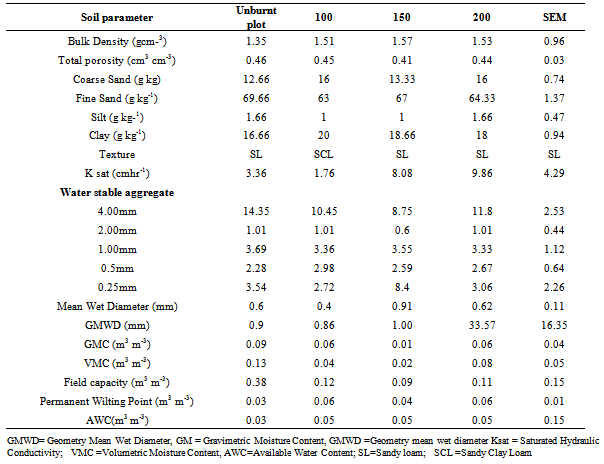
- The physical properties of the study area as shown in Table 1 are grouped based on different levels of dry biomass per square meter soil (Kg DM m-2)
3.1.1. Particle size Distribution
- The textural class of all the soils within the study area was found to be sandy loam with sand fraction being the dominant particle size in all locations after burning and was found to have significant effect on the particle size distribution. Coarse sand fraction was higher in the burnt plot with the mean value of 16.00 gkg-1 in 100 Kg Dm m-2 and 150 Kg Dm m-2 while in 200 Kg Dm m-2 it was 13.33 gkg-1 than 12.66 gkg-1 in the unburnt plot. Increase in coarse sand due to burning may results in enhanced permeability, which is detrimental to the plants, as nutrients will be leached from the root zone especially in the study areas with high level of rainfall. Clay fraction was higher in the burnt with a mean fraction of 20.00 gkg-1 in 100 Kg Dm m-2, 18.66 gkg-1 in 150 Kg Dm m-2 and 18.00 gkg-1 in 200 Kg Dm m-2 than the unburnt plot with mean value of 16.67 gkg-1. This finding is in agreement with the report of Chandler et al[15] who stated that intense fire (> 40℃) permanently alter soil texture by aggregating clay particles into stable sand size particle thereby making the soil texture more coarse and erodible. Although these soils are also characterized by relatively low quantity of silt and clay contents, the low content of silt and clay reflects subjection of the soils to some degree of leaching, water erosion and the sources of the parent materials[16,17].
|
 | Figure 2. Showing changes in soil organic carbon and Nitrogen stock at heating temperature between 30-65℃ at the surface soil |
3.1.2. Bulk Density and Total Porosity
- Intense burning resulted in a significantly high bulk density (1.57 gcm-3 ) which is statistically similar to the effect produced as a result of light burning but different (p < 0.05) from the bulk density of un-burnt areas which gave a mean value of 1.35 gcm-3 within the study. The result is in agreement with the finding that increase in bulk density of the burned soil is as a result of loss of organic matter and destroyed soil structure which lead to increase in the bulk density of the soil. Total porosity decrease on the average from 0.46 - 0.41 cm3 cm-3 after burning. Vegetation burning and long-term cultivation tends to lower total porosity because of a decrease in SOM and large peds[18]Saturated hydraulic conductivity (Ksat) in the burned plots increase from 3.36 cm/hr (control) to 8.08 and 9.86 cm/hr at 150 and 200 Kg Dm m-2 level of burning respectively. Highest saturated hydraulic conductivity noticed in 200 Kg Dm m-2 treatment level could be linked to higher intensity of burning. According to Lal[19], decrease in hydraulic conductivity enhances accumulation of water on soil surface and thereby encourages surface runoff, whereas high value of saturated hydraulic conductivity is an indication of high rate of water movement in the soil which is due to the presence of organic matter content and more capillary pore spaces in the soil[20] Bulk density has been shown to increase[21] during burning, the relationship of bulk density to key processes such as infiltration, gas exchange and root penetration has been used as an indicator of soil quality[22]. Therefore, consistence with change in bulk density, saturated hydraulic conductivity measurements also has demonstrated that there was a significant change in the ability of these soils to transmit water (Table 1). Also, Arthropod burrows reportedly make up a small portion of the total soil volume, but their relative large size and connectivity make them important conduits for water movement. Hence, the relative rapid increase in Ksat with high bulk density in the 150 and 200 Kg Dm m-2 in the burnt plots could be attributed to the presence of more Archoplophora rostralis after burning (Table 2).
3.1.3. Water Stable Aggregate
- Aggregate stability was assessed in the order of 4, 2, 1, 0.5 and 0.25mm sizes. Unburnt plot have a higher aggregate value of 14.35 % (size > 2 mm), which later reduce to 10.45, 8.75, and 11.80% at various level of 100, 150, and 200 Kg Dm m-2 heating respectively. Reduction in aggregation after burning revealed alteration and decrease in soil aggregate stability after burning which leads to erosion[23]. Although arthropods are known to impact macroaggregates and micro-aggregates stability, reduction in arthropod populations resulting from vegetation burning further compound the problem. Impacts of burning were not significant on aggregates < 4 mm. There were significant changes in MWD and GMWD after burning.Aggregate stability is considered one of the most important properties affected by arthropod[24] and impacts important soil processes such as erosion, nutrient cycling and water infiltration. It could be seen in the unburned plots that arthropods products generally help to build more soil aggregate than in the burnt plots. Even though the totality of aggregates in burned plots was 34% higher than the unburned, it may not stand the impact of rain drop. The results of this work is in agreement with the report of Olfert[25], the stability of soil aggregate in soil predominately compose of arthropod oftentimes is greater than stability of similar soils not influenced by arthropod activity “such as in burnt plots”.
3.1.4. Moisture Contents
- Moisture content of the entire plots was generally low. This was due to the sampling period being the dry season. Soil water characteristics as shown by volumetric moisture in Table 1, observed difference in moisture retention before and after burning the field. The amount of moisture content from one landscape position to the other varied considerably with differences in particle distribution. Only small amount (13%) of moisture was retained in the un-burnt plot. Volume of water retained in the soil after burning was not significantly different (p>0.05).After burning, Available water content (0.05 m3 m-3) varied not with the three levels of treatment; 100 Kg Dm m-2, 150 Kg Dm m-2 , 200 Kg Dm m-2 . As expected, the soil tends to store relative more atmospheric moisture for plants use after burning the plots than in unburnt condition. This is so because aggregates have been shown to increase water holding capacity, infiltration and porosity, and reduce compatibility after burning[26].As shown in Fig. 2, there was statistical significant P<0.05 change in soil organic carbon stock and was significantly higher at heating temperature between 30-52℃ at the surface soil. Soil organic carbon stock was completely removed from the soil surface when the heating temperature increases to 65℃ while total nitrogen stock varied at different temperature. Unburnt plot shows significant (P<0.05) different between total nitrogen stock and more total nitrogen content was observed on heating the soil.
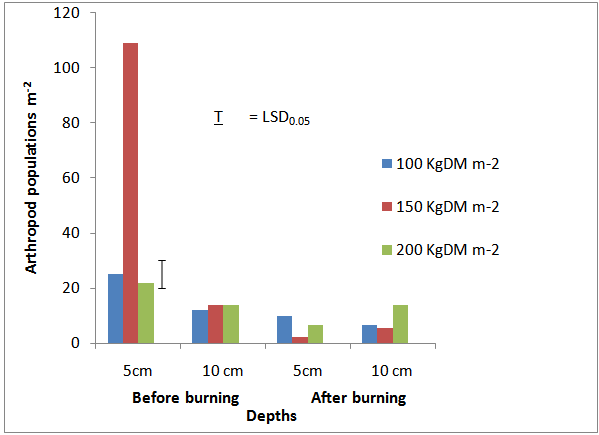 | Figure 3. Mean Arthropod density (individual m-2 ) at three levels of burnt biomass on surface soil |
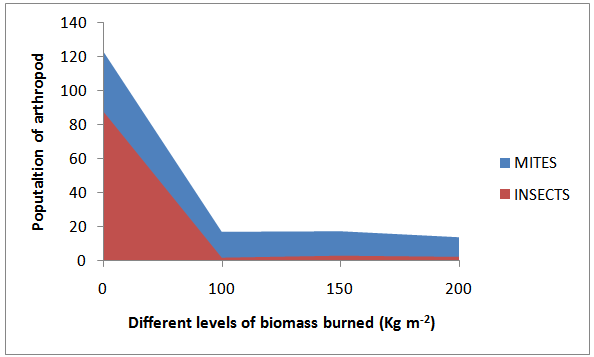 | Figure 4. Effects of burning intensity on arthropod poulation in acid sand soils |
3.2. Arthropod Population Density
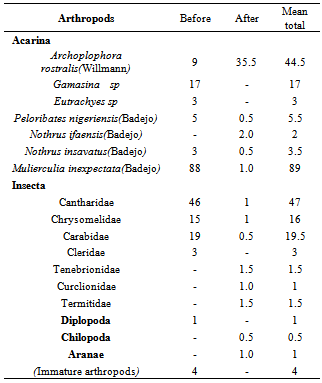
- Five classes of arthropods Acarina, Insecta, Diplopoda, Chilopoda, and Aranaee (Table 2), were isolated from the soil samples. Mean Acarina density before vegetation burning ranged from nil to 88 individuals m-2 soil area and from nil to 35.5 individuals m-2 soil area after burning in the study area. Interestingly, Archoplophora rostralis (Willmann) increased above 74% after burning and non of Gamasina sp and Eutrachyes sp. survived the heat from burning. While Insecta population density varied from nil to 46 individuals m-2 and nil to more than an individual m-2 before and after burning respectively, families of Insecta reduced from nil to 98%, except for the families of Tenebrionidae Curclionidae, and Termitidae. The classes Chilopoda, and Aranaea were only found in the burnt plots. A mean total of 164.5 and 96 individuals arthropods m-2 were isolated from the un-burnt and burnt soils respectively. More than 88 % of the arthropods were either destroyed or burrowed beyond 10 cm depths during the burning. Heat resulting from burning 100, 150 and 200 Kg DM m-2 affected 60, 98, and 70 % of the arthropods respectively at 5 cm depth, whereas at 10 cm depth, the differences were 45, 60 and < 1% respectively across the field (Fig. 3).
|
4. Conclusions
- As expected, the population of arthropod including Gamasina sp; Eutrachyes sp, Peloribates nigeriensis, Nothrus ifaensis, Nothrus insavatus, Mulierculia inexpectat, significantly reduced after burning the farmland, but surprisingly, Archoplophora rostralis outnumbered its population density before burning. High fire temperature and low moisture contents likely combined to reduce the arthropod activity within the rooting zone. A longer time period may be require to detect the impact of increase arthropod populations after burning on bulk density, aggregate stability and Ksat. Our work suggests that disturbance of the physical conditions of the soil with fire, rather than food availability, may control arthropod populations in humid tropics agroecosystem. And this would further decrease the biotic activity of breaking down large amount of soil organic matter through digestion and supplying nutrient-rich excretion.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML