| [1] | Williamson C. E., Dodds W., Kratz T. K. & Palmer M. A. Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Frontiers in Ecology and the Environment, 2008, 6: 247-254. |
| [2] | Sommaruga R. The role of solar UV radiation in the ecology of alpine lakes. Journal of Photochemistry and Photobiology, 2001, B: Biology 62: 35-42. |
| [3] | Vollenweider, R.A. Scientific fundamentals of the eutrophication of lakes and flowing waters, with particular reference to nitrogen and phosphorus as factors in eutrophication OECD. Paris. Tech Report DA 515C116827, 1968, 250 p. |
| [4] | Prescott, G.W. Some relationship of phytoplankton to limnology and aquatic biology. Publ. Amer. Assoc. Adv. Sci. publ., 1939, 10, 65-78. |
| [5] | Lund, J.W.G. Phytoplankton from some lakes of northern Saskatchewan and from Great Salve Lake. Can. J. Botany, 1962, 40: 1499-1514. |
| [6] | Brook, A.J. Planktonic algae as indicators of lake type with special reference to the Desmidiaceae. Limnol. Oceanogr. (1965), 10, 403-211. |
| [7] | Adoni A. D. Work book on limnology. Pratibha Publishers, Sagar, (1985), 1-126. |
| [8] | APHA. Standard methods for the examination of the water and waste water. 20th addition. American Public Health (1998). |
| [9] | Ward H.B. and Whipple G.C. Freshwater biology. (2nd Ed) John Willey and Sons Inc. New York, 1959. |
| [10] | Desikacharya T.V. Book of Cyanophyta. ICAR, New Delhi, Publication, 1958. |
| [11] | Needham J.G. and Needham P.R. A guide to the study of fresh water biology. Publishers-Holden -Day, Inc., San Francisco, U.S.A., 1962, pp: 107. |
| [12] | G.W. The Fresh Water Algae. Brown Company Publishers, Dubuque, Lowa, 1973. |
| [13] | Pennak, R. W. Freshwater invertebrates of United States 2nd Edn. John Willey Sons Inc., New York, 1978. |
| [14] | Victor, R. and Fernando, C. H. The fresh water Ostracoda (Crustacea: Ostracoda) of India. Records of the zoological survey of India, 1979, 74(2) 147-242. |
| [15] | Michael, R.G. & Sharma, B.K. INDIAN CLADOCERA. (Crustacea: Branchiopoda: Cladocea). Fauna of India and adjacent countries. Zool. Sur. India, 1988, p 261. |
| [16] | Edmondson, W. T. Freshwater biology. 2nd Ed. John Wiley & Sons, New York, U.S.A., 1959. |
| [17] | Battish, S.K. Freshwater zooplankton of India. Oxford and IBH Publishing Co. Ltd. New Delhi, 1992. |
| [18] | Reddy, Y.R. Copepoda: Calanoda: Diaptomidae: Guide to the identification of the mio-inverte -brates of the continental waters of the world, 1994, Vol.5 SPB Publishers, The Hague, Netherland. |
| [19] | Sinha S. and Naik M.L. Phytoplankton and Macrophytes in the ponds of Raipur city area, M.P. Pub. Ravi shanker Shucla University, Raipur, M.P., 1997, pp-164. |
| [20] | Sharma, B. K. Freshwater Rotifers (Rotifera: Eurotatoria) Zoological Survey of India. State Fauna Series 3, Fauna of West Bengal, 1999, Part 11: 341-468. |
| [21] | Dhanapathi, M.V.S.S.S.. Rotifers from Andhra Pradesh, India–III. Hydrobiologia, 2003, 48(1): 9-16. |
| [22] | Jaccard, P. "Étude comparative de la distribution florale dans une portion des Alpes et des Jura". Bulletin de la Société Vaudoise des Sciences Naturelles, 1901, 37: 547–579 |
| [23] | S. K., George M.P., Saxena R., Johri M. and Shrivsatava M. Seasonal variation in the limno chemical characteristics of Mansarovar reservoir of Bhopal. In: S.R. Mishra and Saksena, D.N.(eds), Aquatic Ecology, 1992, Ashish Publishing House, New Delhi, pp 275-292. |
| [24] | Agarkar M.S., Goswami H.K., Kaushik S., Mishra S. N., Bajpai A.K. and Sharma U.S.. Biology, conservation and management of Bhoj Wetland 1. Upper Lake Ecosystem in Bhopal. Bionature, 1994, 14 (2), pp 1119. |
| [25] | Wanganeo A., Wanganeo R. and Pani S. Summer dissolved oxygen regimes in tropical Vindhyan Lake in relation to its conservation strategy. Strategy Bionature, 1997, 17(1): 7-11. |
| [26] | Lee G.F., Jones R. A., and Rast W. Alternative approach to trophic status classification for water quality management. Occasional paper no. 66. Dept. of Civil Eng. Env. Eng. Progress. Colorado, State University fort Collins Co., 1981. |
| [27] | Kumar P., Wanganeo A. and Sonaullah F. A Preliminary Limnological Study on Shershah Suri Pond, Sasaram, Bihar. Asian J. Exp. Sci. 2010, Vol. 24, No. 2, 219-226. |
| [28] | Bamforth S. Ecological studies on the planktonic Protozoa of a small artificial pond. Limnology of Oceanography, 1958, Vol. 3, pp. 398-412. |
| [29] | Wanganeo A., Raina R. and Zutshi D.P. Limnological studies on dimictic Himalayan Lake. Rec. Adv. Fish Ecol. Lim. Eco-conserv. 1996, IV: 37-54 |
| [30] | Moyle J.B. Some indices of lake productivity trends. American Fisheries Society, 1946, 76, pp. 322-334. |
| [31] | Raina R., Wanganeo, A., Fozia S. and Pramod K. Variation in summer Limnological characteristics of Bodsar wetland over a period of more than two decades. J. Himalayan Ecol. Sustian. Dev., 2009, Vol 4. |
| [32] | Olsen S. Aquatic plants and hydrospheric factor, I. Aquatic plants in Switzerland, Arizona. J. Sevensk. Botanisk Tidskriff, 1950, 44: 1-34. |
| [33] | Kaul V., Trisal C. L. and Handoo J. K. Distribution and production of macrophytes in some water bodies of Kashmir. In Glimpses of Ecology, 1978, Eds. J.S. Singh & B. Gopal. Praksh Publ. Jaipur. 592 pp. |
| [34] | Wetzel R.G. Limnology. W.B. Saunders Co. Philadelphia, 1975, pp: 743. |
| [35] | Mir A. R., Wanganeo A., Yousuf A. R. and Wanganeo R. Plankton dynamics in relation to fish in Wular lake of Kashmir. Poll. Res. 2007, 26 (4): 733-743. |
| [36] | Wanganeo A. and Wanganeo R. Variation in Zooplankton population in two morphologically dissimilar rural lakes in Kashmir Himalaya. Nat. Acad. Sci. 2006, 76 (B) III. 222-239. |
| [37] | Wanganeo A. and Wanganeo R. Algal population in valley lakes of Kashmir Himalaya. Arch. Hydrobiol., 1991, 131(2): 219-233. |
| [38] | Raina R., Subla B. A. and Zutshi D.P. Water quality and plankton of Jhelum River. Int. J. Ecol. Environ. Sci. 1982, 8: 11-17. |
| [39] | Vass K.K., Wanganeo A., Raina H.S., Zutshi D.P. and Wanganeo R. Summer limnology and fisheries of high mountain lakes of Kashmir Himalayas. Arch.Hydrobiol, (1989): 114-4, 606-619. |
| [40] | Wanganeo A. Phytoplankton photosynthesis, nutrient dynamics & trophic status of Manasbal Lake, Kashmir. 1980, Ph.D. Thesis, Kashmir University (Unpublished). |
| [41] | Raina, R. Plankton dynamics and Hydrobiology of Bod-Sar Lake, Kashmir. 1981, Ph. D. Thesis. Kashmir University, Kashmir, (Unpublished). |
| [42] | Thunmark S. Die Abwassserfrage der vaxijaseen in hydrobiologischer Beleuchtung, Grundzuge in der regination planktologie van sudschweden. Medd. Lunds. Univ. Limnol. Inst., 1945, 4: 239. |
| [43] | Berzins B. Zye limnologie der seen sudosttettlands. Schweiz.Z. Hydrol.1949, 11: 583-607. |
| [44] | Arora H.C. Rotifers as indicators of trophic nature of environments. Hydrobiol. 1966, 27: 46-49. |
| [45] | Sharma B. K. Assessment of pollution indicators in Indian rotatoria. J. Meghalaya Sci. 1986, X., 47-49. |
| [46] | Kulshrestha S. K., Adholia U.N., Bhatnagar A., Khan A.A., Saxena M. and Baghail M. Studies on pollution on river Kshipra: Zooplankton in relation to water quality. Int. J. Ecol. Env. Sci. 1989, 15:27-36. |
| [47] | David A. and Roy S. Studies on the pollution of the river Dana, North Bihar by Sugar and Distillery wastes. Environ. Hlth. 1966, 8: 6-35. |
| [48] | Sharma B. K. The Indian species of the genus Brachionus (Eurotatoria: Monogononta: Brachionidae). Hydrobilogia, 1983, 104, pp 3139. |
| [49] | Kumar Pramod, Wanganeo A, Wanganeo R. and Sonaullah Fozia. Seasonal variations in Zooplankton Diversity of Railway Pond, Sasaram, Bihar. International Journal of Environmental Sciences. 2011, Vol. 2, No 2. |
| [50] | Saxena D.N. and Sharma S.P. Zooplankton fauna of some lentic water bodies of Gwalior.I, Govind sagar, Ehanattri tank, Sawarkar sarover and Matsya sarover. Env. India. 1981, 4:13-17. |
| [51] | Subbamma D. V. Plankton of a temple pond near Machili Patnam, Andhra Pradesh. J. Aqua. Biol., 1992, 7(1 & 2):17-21. |
| [52] | Pejaver M. K. and Somani V. Crustacean Zooplankton of Lake Masunda, Thane. Journal of Aquatic Biology, 2004, 19 (1): 57-60. |
| [53] | Deevey E. S., Deevey Jr. G. B. and M. Brenner. Structure of zooplankton communities in the Peten Lake, District Guatemala. In W. C. Kerfoot (ed.), Evolution and Ecology of Zooplankton Communities. 1980, Pp. 669-678. University Press of New England, Hanover, NH. |
| [54] | Elmore J. L. Factors influencing Diaptomus distributions: An experimental study in subtropical Florida. Limnology and Oceanography, 1983, 28: 522-532. |
| [55] | Lee C. E. and Bell M. A. Causes and consequences of recent freshwater invasions by saltwater animals. Trends in Ecology & Evolution, 1999, 14: 284-288. |
| [56] | Reid J. W. Arctodiaptomus dorsalis (Marsh): A Case History of Copepod Dispersal. Banisteria, 2007, Number 30, pp 3-18. |
| [57] | Ayoade A. A., Agarwal N. K. and Chandola- Saklani A. Changes in Physicochemical Features and Plankton of Two Regulated High Altitude Rivers Garhwal Himalaya, India. European Journal of Scientific Research. 2009. |
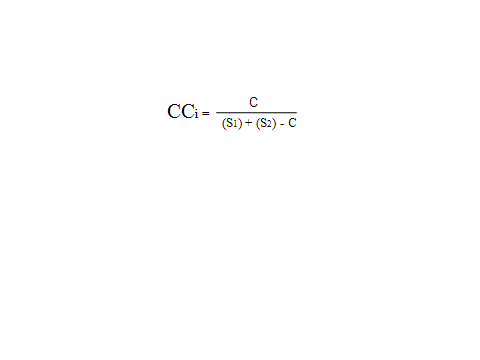 Where,CCi = Jaccard coefficient of community similarity S1 = Number of species present in community 1S2 = Number of species present in community 2C = Number of species common in both the communities
Where,CCi = Jaccard coefficient of community similarity S1 = Number of species present in community 1S2 = Number of species present in community 2C = Number of species common in both the communities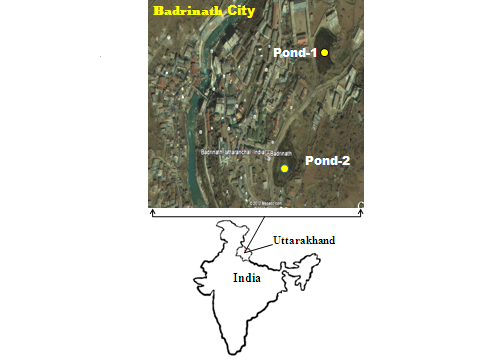
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML