-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2012; 2(5): 93-98
doi: 10.5923/j.ije.20120205.02
Removal of Seeds of Exotic Jackfruit Trees (Artocarpus Heterophyllus, Moraceae) in Native Forest Areas with Predominance of Jackfruit Trees in the Duas Bocas Biological Reserve, Southeastern Brazil
Milena Mileri 1, Marcelo Passamani 2, Frederico Eutrópio 3, Aliny Oliveira 1
1Laboratório de Ecologia e Conservação da Biodiversidade - LECB, Mestrado em Ecologia de Ecossistemas, Universidade Vila Velha, Rua Comissário José Dantas de Melo, 21, Boa Vista, 29102-770 Vila Velha, Espírito Santo, Brazil
2Laboratório de Ecologia e Conservação de Mamíferos, Setor de Ecologia, Departamento de Biologia, Universidade Federal de Lavras, 37200-000, Lavras, Minas Gerias, Brazil
3Laboratório de Microbiologia Ambiental e Biotecnologia - LMAB, Doutorado em Ecologia de Ecossistemas, Universidade Vila Velha, Rua Comissário José Dantas de Melo, 21, Boa Vista, 29102-770 Vila Velha, Espírito Santo, Brazil
Correspondence to: Milena Mileri , Laboratório de Ecologia e Conservação da Biodiversidade - LECB, Mestrado em Ecologia de Ecossistemas, Universidade Vila Velha, Rua Comissário José Dantas de Melo, 21, Boa Vista, 29102-770 Vila Velha, Espírito Santo, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study examined the removal of seeds of the exotic jackfruit Artocarpus heterophyllus (Lamarck) by mammals in a native forest area with predominance of jackfruit trees in the Duas Bocas Biological Reserve, Southeastern Brazil. The seed removal experiments were made in an area with the predominance of jackfruit trees and in an native forest between January and October 2010. At each sampling area three distinct treatments (control: seeds that can be accessed by any species of mammals; semi-protected: seeds that can be accessed only by species of small mammals; and protected: seeds that can not be accessed by any species of mammals) were displayed containing seeds of A. heterophyllus. The area predominated by jackfruit trees showed a greater removal of seeds (35.6%) compared to the native forest (21.6%). The results showed that there was no significant difference in the amount of seeds removed from each area in the protected and control treatments. However, there was a higher seed removal in the semi-protected treatment in jackfruit trees area. In the native forest the removal was significantly higher in the control than in the other treatments. In the jackfruit trees area, the removal of seeds in the control was significantly higher than in the protected treatment. Of the species recorded by camera traps, Trinomys paratus can be considered the main disperser in the jackfruit area, contributing to the dispersal of A. heterophyllus, an invasive plant species. Thus, a management plan for A. heterophyllus at the Duas Bocas Biological Reserve should be carried out partially, gradually and slowly, so that the populations of vertebrate species that now depend on this resource for food do not suffer a drastic impact.
Keywords: Invasion Species, Jackfruit, Seed Remove, Mammals Dispersion, Atlantic Forest
Article Outline
1. Introduction
- Species of small mammals have an important role in the environments through predation and seed dispersal that together are major determining factors in the recruitment of new plants[1, 2]. The dispersal of individuals can be influenced by variation in the abundance and distribution of food resources[3] which, in turn, are influenced by the seasonality of the climate[4]. It is estimated that in tropical forests from 50% to 90% of all trees are dispersed by animals (zoochory), while about 20% to 50% of bird and mammal species consume fruits during, at least, part of the year[5]. Some authors cite rodents as essential predators and dispersers of medium and large seeds of trees[6-9]. Currently,the role played by small rodent communities as seed predators remains neglected, with few studies, since the abundance of these individuals and their dispersal are affected by a myriad of factors such as seed size[10], availability and location of fruits[11], availability of nests[12], habitat characteristics[13, 8], time of year[6], abundance of rodent predators[14], habitat size and hunting pressure[9].Artocarpus heterophyllus Lamarck is a species native to the tropical forests of India, growing between altitudes of 450-1200 m. In Brazil, a well-developed jackfruit tree produces up to 100 fruits that may weigh up to more than 30 kg, fructifying especially in summer (December-February), with the main consumers being man and other mammals like rodents and primates[15]. One of the ecological effects caused by populations of this exotic species is that they grow in dense concentrations, excluding other species and thus altering the local biodiversity[16]. Moreover, this species is considered by many conservation unit managers as an invasive species in the region of the Atlantic Rainforest, causing them to control its population by using girdling techniques and other exclusion methods[17]. According to reference[16] many species native to the Atlantic Rainforest are probably being excluded because of the invasion by the species A. heterophyllus. Thus, the aim of this study was to evaluate the role of mammals to removal and seed dispersal of an exotic species Artocarpus heterophyllus, both in native forest areas and in areas dominated by the jackfruit in Duas Bocas Biological Reserve, southeastern Brazil.
2. Materials and Methods
- Study AreaThe study was made in Duas Bocas Biological Reserve (REBIO), an area of Atlantic Rainforest[18] located in the state of Espírito Santo, southeastern Brazil (20°18’05”S 40°28”06”W; Figure 1). It has an area of 2.910 ha with altitudes ranging between 200 and 738m, and the climate is Tropical wet and dry AW[19]. The area consists mostly of forests in good state of conservation, but there are areas of secondary vegetation that is quite disturbed and in an advanced stage of regeneration. There is also an extensive area inhabited by adult individuals of the exotic species of jackfruit (Artocarpus heterophyllus) in intense reproductive activity and displaying the dynamics of a regenerating population. This area dominated by A. heterophyllus occupies a total of 19.6 ha which represents 0.7% of the total area of the REBIO[20].
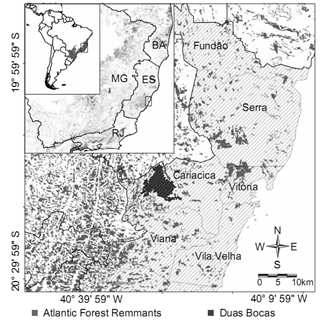 | Figure 1. Map of the state of Espírito Santo, Brazil, showing the Duas Bocas Biological Reserve |
3. Results
- Of the 1800 seeds of A. heterophyllus used in the experiment, 514 (28.6%) were completely removed. In the area with a predominance of jackfruit trees, 35.6% of seeds were removed and in the area of native forest, 21.6%.The comparison of each treatment between the two sampled areas showed that there was no significant difference in the amount of seeds removed from each area in the protected and control treatments (t =- 1.236, p = 0.218, and t = -1.820, p = 0.071, respectively). However, there was a higher seed removal in the semi-protected treatment in the area with the predominance of jackfruit trees, compared with the area of native forest (t =- 3.233, p = 0.001, Figure 2).
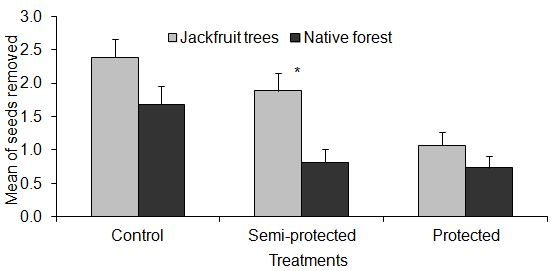 | Figure 2. Mean of seeds removed among treatments in two areas studied. The bars indicate the means and standard deviation of seeds removed |
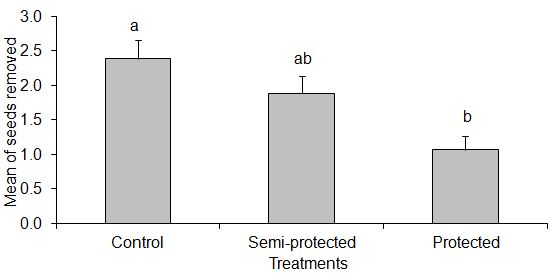
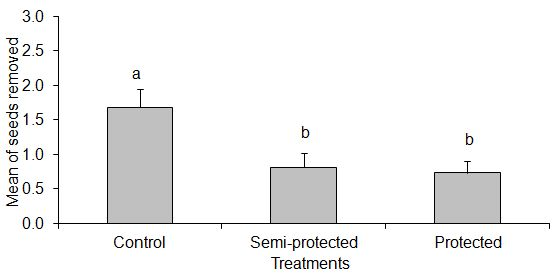 | Figure 3. Mean of seeds removed among treatments in the native forest. The bars indicate the means and standard deviation of seeds removed. Different letters represent a significant difference |
 | Figure 5. Photographic record of T. paratus with seeds of A. heterophyllus of the experiment in its mouth |
4. Discussion
- In general, the highest rates of removal of A. heterophyllus seeds occurred in areas with a predominance of jackfruit trees, especially in the semi-protected treatment, which only allowed small species of mammals to access the seeds. Areas with high density or provision of resources are easily found by animals, which makes the use of these areas by individuals different from the consumption of other species with a more uneven distribution and with a lower supply of resources[22]. Based on this, the area with a predominance of jackfruit trees in REBIO presents a wide supply of resources, which promotes greater removal rate of seeds by individuals. Studies in forests have assigned high seed removal rates to small mammals[23-27], which are also important seed dispersers[28, 29]. Small rodents are mostly considered seed predators[30] and may limit population growth of certain plants if the amount of seeds that are dispersed is insufficient or if the quality of the dispersal is inadequate[31]. Seed predation by small rodents is an important factor of seed mortality in the Neotropics[32]. However, it can contribute to the increase in the establishment of seedlings for germination in other potential sites away from the mother plant[33-35]. The absence of a significant difference between the control and semi-protected treatments in the jackfruit area indicates that small mammals, especially T. paratus, may have been the main dispersers of A. heterophyllus seeds in the area. These results are reinforced by the photographic records, since T. paratus was the species most often recorded by the camera traps (more than 40% of the records). Reference[36] found that on islands of Panama the other species of spiny rats (genus Proechimys) buried seeds of the palm tree Astrocaryum standleyanum, acting as effective secondary seed dispersers[37]. Similar to verified in this study, the rodent Oryzomys oniscus was the most responsible for seed predation in northeastern Brazil[38], and like T. paratus may operate as an effective large-seed predator/disperser in forest.Conversely, significantly higher removal rates in the control treatments when compared with the protected and semi-protected treatments in native forest areas, point to the fact that medium and large mammals are also important groups in the removal of seeds in native areas of the REBIO. Similar results were found by Reference[39] at the Caetetus Ecological Station (SP), where the seeds in the open (control) treatment were removed three times more than in treatments that only small mammals and invertebrates could access. Reference[14] found that unprotected seeds in the Los Tuxtlas Biological Station (Mexico) experienced a dramatic risk of predation, with levels ranging from 50 to 80%. In according to reference[40] rodent species may be operating as active seed predators in fragments of Atlantic forest. The various photographic records of specimens of T. paratus with A. heterophyllus seeds in its mouth suggest that this species acts as a disperser, since it takes the seeds away from the adult plant and probably buries them as do the other spiny rats of the genus Proechimys[36]. According to Reference[9] and[41] small rodents of the genus Trinomys spp. consume fruits and seeds of many Atlantic Forest species, being considered good seed dispersers since they take them far away from the adult plant. Thus, it seems that T. paratus is the main disperser of A. heterophyllus removing most of the seeds and contributing decisively to the expansion of jackfruit trees at the Duas Bocas Biological Reserve.Data from the camera traps reinforce the hypothesis that small mammals are removing most of the seeds of A. heterophyllus. Among the species considered potential seed dispersers recorded by camera traps is Dasyprocta agouti that consumes / disperses seeds of the Acuri (Attalea phalerata). Agoutis (genus Dasyprocta) display a behavior in which they bury few seeds in different locations. This strategy would be more efficient for dispersal, because animals cannot return to all the sites where they have stored seeds, increasing the chance of recruitment[42].According to Reference[16], while exotic species are rare in undisturbed tropical forests, many species can become invasive and even cause drastic changes to the ecosystem, facilitating their own dominance over native species. Similar to that seen in this study at the Duas Bocas Biological Reserve, the invasion and prevalence of extensive areas of jackfruit (A. heterophyllus) was also observed in the Parque Nacional da Tijuca in Rio de Janeiro[16]. When direct effects caused by the invasive species are not noticed, the population control is not usually considered an alternative. In this way, gradually as the jackfruit populations colonize the environment and slow growth typical of native tree species[43], the effects caused by these plants in the ecosystem may go unnoticed for a long time. However, for the Duas Bocas Biological Reserve these effects are noticeable[20] and management programs for this species should be planned and implemented. Issues related to species conservation through management strategies are often more complex than the simple remediation of the primary cause[44]. Interventions should take into account the new ecological interactions that have developed and how the removal of the species would affect the local physical environment[16]. Thus, the removal of this exotic jackfruit species (A. heterophyllus) in the Duas Bocas Biological Reserve should be performed, however, the results of this study point to some facts that should be taken into account. The additional supply of jackfruit fruits and seeds allows many species of mammals to increase their populations in this area. This is easily verified by the high abundance of T. paratus rodents in these areas[45], the large number of photographic records obtained of various species of mammals and the frequent encounters with species of medium mammals (Cebus nigritus, Callithrix geoffroyi and Nasua nasua) consuming fruits of this species (unpublished data). Thus, management should be carried out partially, gradually and slowly, so that the populations of vertebrate species that now depend on this resource for food do not suffer a drastic impact.
5. Conclusions
- The results of this study showed that there was a higher seed removal in the semi-protected treatment in the area with jackfruit trees than in native forest, and in native forest the removal was higher in the control than in the other treatments. This demonstrated that medium and large mammals are important groups in the removal of seeds in native areas and that small rodents was in jackfruit area. The species of spiny-rat Trinomys paratus was a potential seed disperser of Artocarpus heterophyllus in Duas Bocas Biological Reserve. The management of the area should be carried out partially, gradually and slowly, so that the populations of vertebrate species that now depend on this resource for food do not suffer a drastic impact.
ACKNOWLEDGEMENTS
- We thank the Graduate Program in Ecosystem Ecology of the University Vila Velha (UVV). We also thank the State Environmental Institute (IEMA) for the collection permit and the Environmental Manager Fabiano Z. Novelli, and staff of the Duas Bocas Biological Reserve.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML