-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
2012; 2(2): 1-4
doi: 10.5923/j.ije.20120202.01
Soil Microarthropods in A Secondary Rainforest, Rivers State, Nigeria - III- Partial Recovery after an Oil Spill
Tambeke N. Gbarakoro 1, Samuel N. Okiwelu 1, Odidika C. Umeozor 1, Adetola Badejo 2
1Department of Animal and Environmental Biology, University of Port Harcourt, P.M.B. 5323 Choba, Port Harcourt, Nigeria
2Department of Zoology, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Samuel N. Okiwelu , Department of Animal and Environmental Biology, University of Port Harcourt, P.M.B. 5323 Choba, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
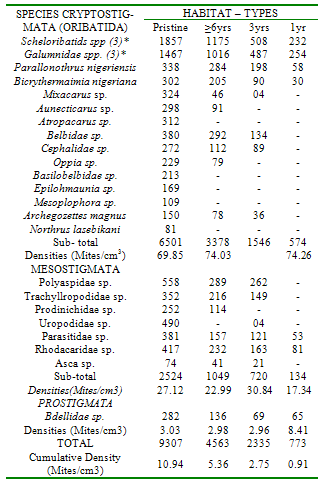
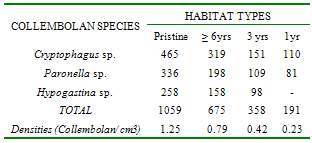
Investigations were undertaken at 4 habitat-types (unpolluted, oil-polluted 1year, 3 and 6 years pre-study) in a secondary rainforest, Nigeria, to determine trends in the natural recovery of soil micro-arthropods (mites and Collembolan) in the polluted habitats. Soil samples were taken monthly with an 8.5cm-diameter bucket-type auger over a 2yr-period (May, 2007 - April, 2009). Extraction was by the Berlese-Tullgren funnel. Identification was conducted with the aid of standard keys and comparisons of unidentified species with type specimens. Mean Total Hydrocarbon (THC) values were 10mg/kg (undisturbed) 630mg/kg (polluted 1 year pre-study) 260mg/kg (polluted 3 years pre-study) and 125mg/kg (polluted 6years pre-study) habitats. In all habitat-types, Cryptostigmata (Oribatida) were the most abundant mites. Three of the 11 Oribatid species were absent from the habitat polluted, 1-year pre-study but re-colonized the habitat polluted 3 years pre-study, while 4 of the 5 Mesostigmata species re-colonized this habitat. In the habitat polluted 6years pre-study, two more Oribatid species and the Mesostigmata Prodinichidae species re- colonized this habitat. In contrast, approximately 8 years after the oil spill, 5 Oribatid species and the Mesostigmata, Uropopidae spp never recovered. The Collembolan sp Hypogastina, absent from the habitat, polluted 1 year pre-study, re-colonized the habitat, polluted 3 years pre-study. There were significant positive correlations between total mite and collembolan densities and the length of the intervening periods between occurrence of spill and commencement of the study. (Fcal=4.63; Ftab=3.62; P< 0.05; Fcal=7.80; Ftab =3.62; P<0.05). The implications of these results in the bio-monitoring of oil- polluted habitats are discussed.
Keywords: Tropical Forest, Oil Pollution, Soil Microarthropods, Re-Colonization
Article Outline
1. Introduction
- Cryptostigmates (orbatids) are sensitive to environmental changes; they have little capacity for rapid growth and few are adapted for dispersal; they are therefore unable to easily escape environmental stress[1]. The abundance and diversity of Collembola have been widely used to assess the environmental impact of a range of pollutants on soils[2]. The responses of these soil microarthropods (mites- Cryptostigmata, Mesostigmata, Prostigmata; Collembolans) to oil pollution were investigated at 4 habitat- types: unpolluted, polluted by an oil spill 1yr, 3 yr and ≥ 6yr pre-study in a secondary rainforest, Rivers State, Nigeria. Observations from these studies on seasonal variations in species richness, vertical distribution, density in the unpolluted habitats and their role as indicators of ecosystem health have already been published[3,4]. This report focuses on the partial recovery of these soil microarthropods after an oil spill.
2. Materials and Methods
2.1. Study Area
- The habitat - types were located in secondary rainforest, Tai Local Government Area, Rivers State, Nigeria. Two of the habitats, undisturbed, (04o 44´15N; 007o12´ 32E) and polluted (3 yrs pre-study (04o 44´14N; 007o12´ 29E) were located at Norkpo; the habitat polluted 1 yr pre-study was at Kporghor (04o42.98N; 007o12´ 98E) and the habitat polluted ≥ 6 yrs pre-study was at Gio (04o41.91N; 007o14´ 85E).
2.2. Methods
- A 3500m2- area was demarcated within each habitat for study. The unpolluted and polluted habitats were each divided into twelve 24x12m sub-plots to ensure total coverage during sampling over a 2-year period, May, 2007-April, 2009. Soil samples were taken monthly with an 8.5cm- bucket type auger at the southern section of each sub-plot during the first year, May 2007-April 2008. In the second year, May 2008-May 2009, samples were taken from the northern section. Depths of soil collections were: 0-5.0cm, 5.1-7.5cm, 7.6-10.0cm. 10.1-12.5cm 1nd 12.6-15.0cm.To ensure that the entire soil in each range was collected, the auger was rotated clockwise and anti-clockwise, until the entire soil was taken. Each sample was placed in a plastic bag, labelled and taken to the laboratory for analyses, over a 3-stage process (extraction, sorting, and identification). The modified Bukard model of the Berlese -Tullgren funnel was used for extraction[5]. The extractor complex consisted of two rows of 8 units each, enclosed in a wooden cabinet. Description of the extractor unit and extraction procedure has been documented[6]. The duration of extraction was 7 days. The extracts containing soil microarthropods were placed in Petri-dishes under a dissecting microscope; the mites and collembolans were carefully removed. Temporary slides were prepared and identification undertaken under a compound microscope at the Entomology Research Laboratory, Department of Animal and Environmental Biology, University of Port Harcourt. Identification keys were used [7-9]. Unidentified specimens were taken to the Laboratory of Systematics and Ecology of Microarthropods, Department of Zoology, Obafemi Awolowo University, where they were compared to type specimens.
|
3. Results
- The mean Total Hydrocarbon Content (THC) of soils in the various habitat-types was: unpolluted, 10mg/kg; polluted - 1yr pre-study 630mg/kg; 3yrs pre-study 260mg/kg; 6yrs pre-study 125mg/kg. In all habitat-types, among, the Cryptostigmata (Oribatids) were most abundant (66-74%), followed by Mesostigmata (17-30%). Three (Mixacarus sp., Belbidae sp., Cephalidae sp.) of the 11 Oribatid species found in the unpolluted habitat were absent from the habitat polluted 1yr pre-study but recolonized the habitat polluted 3yrs pre-study. In the Mesostigmata, 4 species (Polyaspidae sp., Trahyllropodidae sp., Uropodidae sp. and Asca sp.) had recolonized the habitat, polluted 3yrs pre-study. In the Oribatids, natural recovery of an additional two species Aunecticarus sp., and Oppia sp.) occurred in the habitat, polluted 6yrs pre-study. In the Mesostigmata, Prodinichidae sp. recolonized the habitat, polluted 6yrs pre-study. However, in the habitat, polluted 6yrs pre-study, 5 species (Atropacarus sp., Basilobellidae sp., Epilohmaunia sp., Mesoplophora sp., and Northrus lasebikani) of Oribatids were absent. Similarly, in the Mesostigmata, Uropodidae sp. was also absent (Table 1). Among the Collembolans, the only species, Hypogastina sp., that was absent in the habitat, polluted 1yr pre-study, recolonized the other two habitats, polluted 3 and 6yrs pre-study (Table 2).There was a significant positive correlation between mite densities and length of the intervening period between occurrence of the spill and initiation of the study (Fcal = 4.63; Ftab =3.62; p<0.05) (Fig 1). There was also a significant positive correlation between total Collembolan densities and the length of the intervening period between occurrence of the spill and initiation of study ((Fcal =7.80; Ftab=3.62; p<0.05) (Fig 2).
|
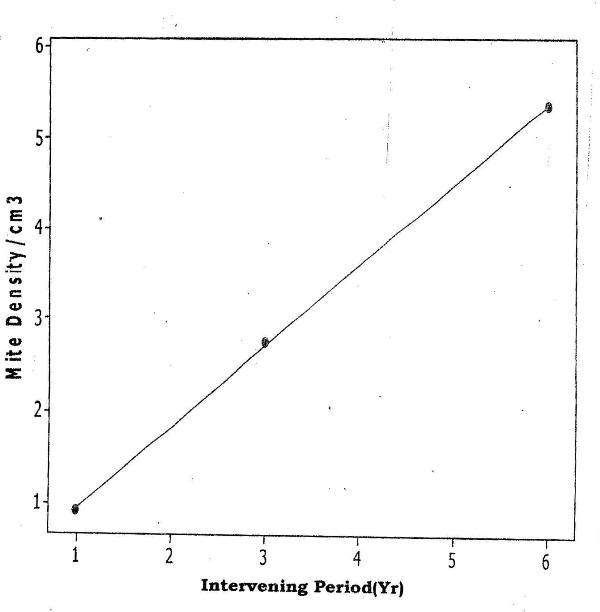 | Figure 1. Relationship of Mite Density to the Length of the Intervening Period from Oil Spill to Commencement of Study |
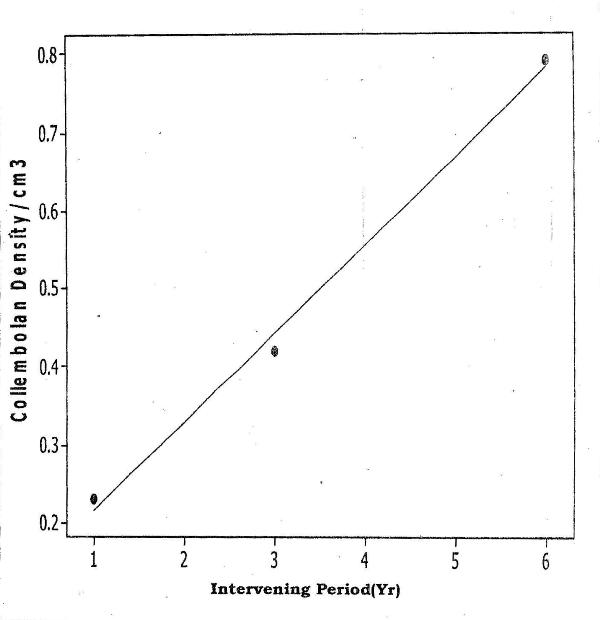 | Figure 2. Relationship of Collembolan Density to the Length of the Intervening Period from Oil Spill to Commencement of Study |
4. Discussion
- Apparently, the most susceptible mites were the five oribatid species (Atropacarus sp., Basilobellidae sp., Epilohmaunia sp., Mesoplophora sp., and Northrus lasebikani) and one Mesostigmata, (Uropodidae sp.) that were eliminated for about 8yrs (6yrs before commencement of the study and 2yrs of study) after the spill. Oribatids have little capacity for rapid growth; few are adapted to dispersal and therefore unable to easily escape environmental stress [1]. Collembolans were apparently not as susceptible as mites to the spill, because all species re-colonized the habitat, where the spill occurred, 3yrs pre-study. The significant reduction in mite and collembolan densities might have been caused by direct and indirect effects. The spill might have killed off many of the microarthropods by adversely affecting respiration. The oil might have adversely affected their food source, leading to reduced reproductive rate. Some microarthropods are fungal feeders (mycophages), while the Collembolans, free-living astigmatid mites and most Oribatids (Cryptosigmata) have well-developed mouthparts capable of fragmenting organic matter adhering to detritus[2]. Since mites and other arthropods which are part of the mesofauna, play a crucial role in the context of soil biodiversity, decomposition and mineralization processes[10,11] in an oil-polluted soil lacks these key members of the soil community.Although the differences in mite and Collembolan densities between the unpolluted habitat and habitat polluted 6yrs pre-study were not significant, the mite and Collembolan densities in the latter were respectively, 49.00% and 63% of the former. Approximately 8 years after the spill, species richness in Cryptostigmats and Mesostigmata mites was also not fully restored. In contrast, species richness was fully restored among Collembolans. The capacity for oribatid assemblages to recover following disturbance has not been well examined, but it is estimated that it could require anywhere from five[12] to thirteen years[13-15] to return to pre-disturbance levels, depending on the severity of the perturbation.Recovery can depend on disturbance frequency, biotope quality (favourable conditions) and re-colonization[16]. Rec-olonization of Oribatid assemblages may be very slow and some may be locally extinct due to their low dispersal ability[16,17], their low fecundity and slow development[1]. Long term monitoring of the recovery of forest biodiversity following disturbance is critical to the assessment of the effectiveness of ecosystem management practices.
5. Conclusions
- Oribatids are very susceptible to oil spills, because approximately 8 years post-spill, five oribatid species had not re-colonized the habitat. Collembolans are not as susceptible because 5 years post-spill, they re-colonized the habitat. The spill adversely affected both abundance and species richness of these microarthropods. Eight years post-spill, re- colonization did not attain pre-spill, re-colonizing species did not attain pre-spill abundance levels
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML