-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Ecosystem
p-ISSN: 2165-8889 e-ISSN: 2165-8919
2012; 2(1): 19-27
doi:10.5923/j.ije.20120201.04
Impact of Altitude on Population Structure and Regeneration Status of Two Rhododendron Species in a Temperate Broad Leaved Forest of Arunachal Pradesh, India
Sanjeeb Bharali1, Ashish Paul1, Mahamed Latif Khan1, 2, Lal Bihari Singha1
1Department of Forestry, North Eastern Regional Institute of Science and Technology (Deemed University), Nirjuli, Arunachal Pradesh, 791109, India
2Department of Botany, Guru Ghasidas University, Koni, Bilaspur, Chhattisgarh, 495009, India
Correspondence to: Sanjeeb Bharali, Department of Forestry, North Eastern Regional Institute of Science and Technology (Deemed University), Nirjuli, Arunachal Pradesh, 791109, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
An attempt had been made to study the impact of altitude on population structure and regeneration status of two Rhododendron species as well as its associated species in a temperate forest of Arunachal Pradesh. Population structure was worked out based on the density of seedlings, saplings and adults while, the regeneration status was determined from the population size of seedlings, saplings and adults. The mid altitude site (Hanuman Camp) has highest density (2692 individuals ha-1) comprising of seedlings, saplings and adults of all species followed by lower altitude (Shagong) site (2379 individuals ha-1) and lowest at (Yarlung) higher altitude site (2180 individuals ha-1). However, the relative proportion of seedlings of all woody species was recorded highest in low altitude site, while it was lowest in higher altitude. Conversely, the relative proportion of saplings and adults were highest in higher altitude. The selected Rhododendron species shows reverse J shaped population structure and fair regeneration status in all study stands having higher number of seedlings compared to saplings. However, the number of saplings is less than the adults. It was observed that the seedling populations dominate the overall population of the selected Rhododendron species. Moreover, it was found that although altitude does not affect regeneration status, but affects the population structure of the selected Rhododendrons. The fluctuation in population density of seedlings, saplings and adults along the altitudinal gradient may be linked with the prevailing environmental factors.
Keywords: Population Size, Biotic and Abiotic factors, Canopy, growth
Cite this paper: Sanjeeb Bharali, Ashish Paul, Mahamed Latif Khan, Lal Bihari Singha, Impact of Altitude on Population Structure and Regeneration Status of Two Rhododendron Species in a Temperate Broad Leaved Forest of Arunachal Pradesh, India, International Journal of Ecosystem, Vol. 2 No. 1, 2012, pp. 19-27. doi: 10.5923/j.ije.20120201.04.
Article Outline
1. Introduction
- Population is a basic community component i.e., the population structure has a direct impact on the community structure, which in turn demonstrates the development trend of the community[1,2]. The population structure of a tree species reflects its biological and ecological characteristics [3] and it refers to the distribution of individuals in different height classes to provide the regeneration profile. Although diameter/girth at breast height (DBH/GBH) is the most commonly used size variable in the analysis of population structure, height class distribution for seedlings and saplings have also been used for the study of population structure of tree species[4-6]. The inclusion of seedlings and saplings in plant population structures would provide better information about the status of the species at early stage ofregeneration. Plants maintain and expand their populations in time and space by the process of regeneration. Population dynamics of plant species can be described by demographic variables such as the recruitment, mortality and growth rates of individuals[7] and the balance among these variables has been found to regulate the dynamics and the structure of a population[8]. Regeneration of any species is restricted to a peculiar range of habitat conditions and the extent of those conditions is a major determinant of its geographic distribution[9]. However, natural regeneration of a tree species largely depends on production and germination of seeds, establishment of seedlings and saplings[10].The pattern of population dynamics of seedlings, saplings and adults of a plants species can exhibits the regeneration profile, which is used to determine their regeneration status[11,12]. A population with sufficient number of seedlings and saplings depicts satisfactory regeneration behaviour, while inadequate number of seedlings and saplings of the species in a forest indicates poor regeneration[13,14]. The density of seedlings and saplings are considered as regeneration potential of a species. Good and Good[15] considered three major components for successful regeneration of a species viz., ability to initiate new seedlings, ability of seedlings and saplings to survive and ability of seedlings and saplings to grow. Moreover, plants could generally grow and survive in a limited range of environmental gradients, e.g. temperature and light availability[16] and variation in these factors play important roles in shaping the age structure and forest regeneration at different altitudes[17]. The population structure is the integrated exhibition of the status, function, development process and trends of tree species[2]. Thus, the regeneration type, dynamic process and trends of the community can be confirmed or speculated according to the characteristics of population structure[3]. Population structure studies along altitudinal gradients of a mountain would be helpful in understanding the influences of environmental factors on the regeneration of natural forests[18].Arunachal Pradesh, by virtue of its geographical position, climatic conditions and altitudinal variations, is a biodiversity rich region in northeast India. Rhododendrons form the dominating species all along the cool temperate, subalpine and alpine zones in Arunachal Himalaya. About 85% of the Indian Rhododendron species are found in Arunachal Himalaya[19]. The rhododendrons in Arunachal Pradesh are also experiencing the impact of disturbance, which is resulted due to the shrinkage of green cover. This phenomenon is clearly visible in the temperate and sub alpine zones of Arunachal Himalaya, where ecosystem and the land physiography are supposed to be fragile and easily disturbed. The major threats to rhododendrons are large-scale deforestation of their suitable habitats and unsustainable extraction for firewood by local people[19]. Moreover, till date no detailed studies on population structure and regeneration status of Rhododendron species with reference to altitude particularly in Arunachal Himalaya have been carried out. Keeping this in view, an attempt had been made to assess the impact of altitude on population structure and regeneration status of two selected Rhododendron species (viz., Rhododendron kenderickii and Rhododendron grande) as well as its associated species in West Siang district of Arunachal Pradesh. R. kenderickii is reported as rare in Arunachal Himalaya[19,20] while, R. grande is threatened in Sikkim Himalaya[21].
2. Materials and methods
2.1. Study site
- Arunachal Pradesh, the largest state in northeastern region of India forms a major part of Eastern Himalaya and is predominantly hilly and mountainous[22]. The state is also recognized as one among the 200 globally important ecoregions[23]. The Rhododendron forest under the present study was located in Mechuka valley in West Siang district of Arunachal Pradesh, northeast India (Figure 1). The West Siang district lies between 27°32' to 28°59' N latitude and 93°58' to 94°58' E longitude. The district is spread over 7,813 sq. km and constitutes 9.33% of the total geographical area of the state. The total forest cover of the district is 6,719 sq. km (86 % of the total geographical area), out of which very dense forest comprises of 2,478 sq. km, moderately dense forest 2,741 sq. km and open forest covers about 1,501 sq. km[24]. Mechuka falls under the Himalayan range and is characterised by rough topography with mountains, deeply incised valleys, escarpments and plateaus. Mechuka is bordered by Upper Siang district in the northeast, Upper Subansiri district in southwest, whereas the northwestern side is bordered by China. Climate of Mechuka can be characterized into four distinct seasons viz. winter with temperature below freezing point when maximum snowfall occurs, spring with little rainfall, rainy with highest rainfall and autumn with little shower. The precise age of the forest of the study area is not known due to non-availability of the forest history with the local forest department. Thick spongy humus layer covers the forest floor. The dominant species of the study area is Rhododendron kenderickii, R. grande, Abies densa, Taxus wallichiana, Pinus wallichiana etc. The forest is in climax stage and very old aged. The soil is coarse textured with sandy to loamy in nature and the pH ranged from 4.08 – 4.71. The organic carbon content ranged from 4.87% – 6.89% while, the total nitrogen, available phosphorus and exchangeable potassium content ranged from 0.15% – 0.25%, 0.007% – 0.01% and 0.37% – 0.43% respectively. The rock types in Mechuka can be categorised into two distinct groups namely Meta-sedimentaries and Gneisses[25].
 | Figure 1. Map showing the study site |
2.2. Methods
2.2.1. Population Structure
- Population structure of all the woody species occurring in each study stands along the altitudinal gradient was studied using quadrat method. Forty quadrats of 10 m x 10 m were laid randomly in each stand. The seedlings were also recorded within the same 10 m x 10 m quadrats as they are sparsely distributed. Species were identified and densities of seedlings (≤ 50 cm in height), saplings (≤10 cm collar circumference at the base and > 50 cm height) and adults (≥ 10 cm girth at breast height, i.e. 1.37 m above ground level) were determined. Specimens of all species were collected and herbariums were prepared following Jain and Rao[26] and identification was done following flora references like Materials for the flora of Arunachal Pradesh, Flowers of Himalaya and Flora of Assam etc. The relative proportion of the seedlings, saplings and adults to total density of tree species in each study stand was calculated.
2.2.2. Regeneration Status
- Regeneration status of species was determined based on population size of seedlings and saplings[27]: good regeneration, if seedlings > saplings > adults; fair regeneration, if seedlings > or ≤ saplings ≤ adults; poor regeneration, if the species survives only in sapling stage, but no seedlings (saplings may be <, > or = adults).
3. Results
3.1. Tree Species Composition
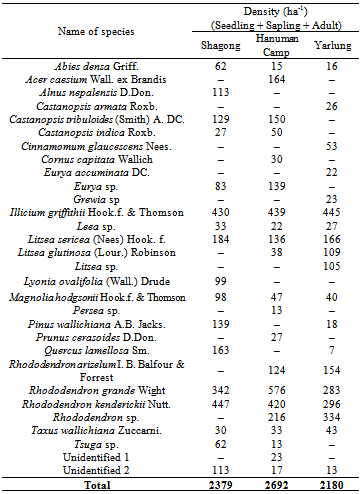
- The number of tree species encountered from the study sites varied from stand to stand. Highest number of tree species (21) was recorded from Hanuman Camp, followed by Yarlung (19) and least number from Shagong (15). Hanuman Camp has highest density (2692 individuals ha-1) of total seedlings, saplings and adults followed by Shagong (2379 individuals ha-1) and lowest at Yarlung (2180 individuals ha-1) (Table 1).
3.2. Population Structure
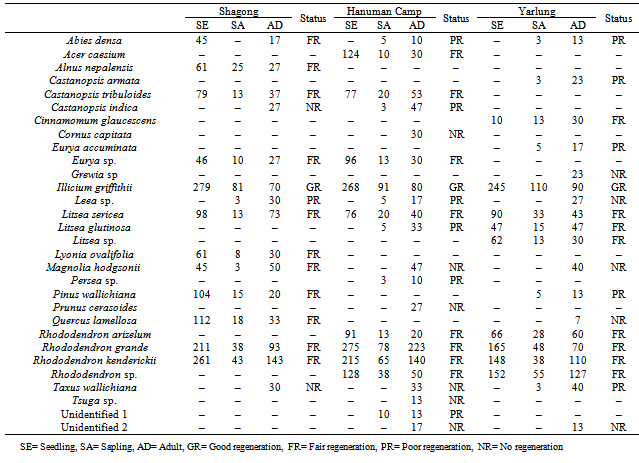
- The population structure of tree species in terms of the proportion of seedlings, saplings and adults varied greatly in the three study stands (Figure 2). The relative proportion of seedlings was recorded highest in Shagong, while, it was lowest in Yarlung. On the other hand, the relative proportion of saplings and adults were highest in Yarlung followed by Hanuman Camp. The distribution of seedlings, saplings and adults along the altitudinal gradient also showed variations among species (Table 2).
|
 | Figure 2. Population structure of tree species in the three study stands |
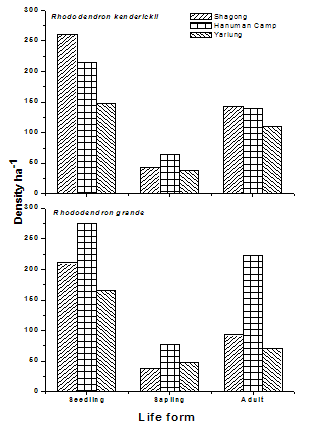 | Figure 3. Variation in density (ha-1) of seedlings, saplings and trees of the selected Rhododendron species in the three study stands |
3.3. Regeneration status
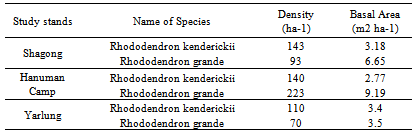
- The selected Rhododendron species (viz., Rhododendron kenderickii and R. grande) shows fair regeneration in all the study stands having higher number of seedlings compared to saplings. However, the number of saplings is less than the adults (Table 2).
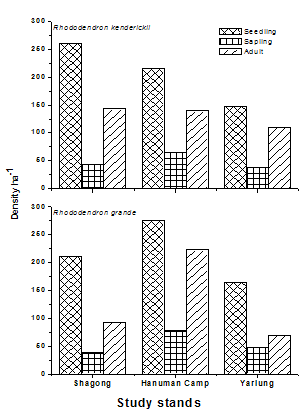 | Figure 4. Population structure of the selected Rhododendron species at the three study stands |
4. Discussion
- The future community structure and regeneration status of the plant species could be predicted from the relative proportion of seedlings, saplings and adults in the total populations of various species in the forest. The overall population structure of tree species in the three study stands showed that contribution of seedlings was highest to the total population followed by adults and saplings. Variation in proportion of seedlings, saplings and adults were observed in all study stands. This differences in relative proportion of seedlings, saplings and adults among the three study stands may be due to the interactive influence of an array of biotic and abiotic factors[28]. According to Jones et al.[29], seedling layer in various forests differs in composition from their respective over storey.The variation among the population size of seedlings, saplings and adults at the three study stands may be due to the variation in climatic as well as edaphic factors required by the selected Rhododendron species for growth and survival. Tasfeye et al.[30] also reported similar variation in seedling density among species along altitudinal gradient in dry Afromontane forest, Southern Ethopia. Inspite of variation in density the selected Rhododendron species (viz., R. kenderickii and R. grande) exhibit reverse J shaped distribution in all the study stands. Paul[31] also reported similar type of distribution from temperate broad-leaved forest of rhododendrons (R. arboreum, R. arboreum ssp. delavayi var. delavayi, R. barbatum and R. kesangiae). Moreover, Miyadokoro et al.[32] from subalpine old-growth coniferous forest, central Japan reported reverse J shaped distribution. Shrestha[33] has also reported that population of Betula utilis both in mixed and pure forests nearly resembled a reverse J-shaped in forest trans-Himalayan dry valley in Manang (central Nepal). Wangda and Ohsawa[34] have reported that three types of population structure i.e. unimodal, sporadic and reverse-J shaped by the dominant species along the altitudinal gradient in dry valley slopes of the Bhutan Himalaya. Duan et al.[17] studied the population structure of Larix chinenesis in Qinling Mountains (China) and reported that population structure in the low altitude transect was closed to bell-shaped and characterized by the dominance of adult trees while, a reverse-J shape age structure was found in the mid-altitude transect. Multi-modal age distribution was found in the high altitude transect, and was caused by lack of young seedlings and saplings. Yang[35] have also reported that the main populations of Badaling forest in Beijing, China can be divided into 4 types of population structures: sporadic type (Quercus mongolica), reverse J type (Tilia mandshurica and T. mongolica), unibar type (Fraxinus rhynchophylla) and ladder type (Betula dahurica and Acer mono). Moreover, Zegeye et al.[36] studied the population structure of woody species in Tara Gedam and Abebaye forests, northwestern Ethiopia and reported that all the woody species shows a reverse J type population structure. Reverse “J” distribution is considered as an indication of stable population structure with fairly good regeneration status[37-39]. Further, it was also reported that the seedling pool of Rhododendron simiarum were abundant and the age structure was stable and population can stably expand in the community. It might be determined by its biological characteristics and the integrated environmental influence[40]. The population structure variations among the selected Rhododendron species in three study stands might be ascribed to the differences in their habitats and existing micro environmental factors[41].
|
|
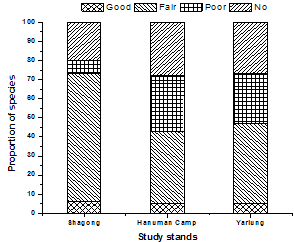 | Figure 5. Regeneration status of tree species in the three study stands |
5. Conclusions
- It can be concluded that, seedling populations in all the three study stands dominate the overall population of the selected Rhododendron species. Moreover, it was found that although altitude does not affect regeneration status of the selected Rhododendron species, but affects the population structure. The fluctuation in population density of seedlings, saplings and adults along the altitudinal gradient may be linked with the prevailing environmental factors. The selected Rhododendron species have fair regeneration status in all study stands as indicated by low population size of saplings compared to seedlings and adult, which may be due to close canopy as well as absence of other favorable environmental factors such as light, temperature, moisture, nutrients etc. for growth and survival.
ACKNOWLEDGEMENTS
- We thank the PCCF, Arunachal Pradesh and Additional Deputy Commissioner, Mechuka for research permit. The corresponding author thankfully acknowledges the financial support provided by University Grant Commission, GOI, through Rajiv Gandhi National Fellowship.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML