Kristanty Randa Arung1, Tjandra Manukbua2, Triapermatasari Buntugayang3, Indra Zachreini4
1Psychiatrist in Lakipadada General Hospital, Tana Toraja, Indonesia
2Otolaryngologist in Lakipadada General Hospital, Tana Toraja, Indonesia
3Psychologist in Lakipadada General Hospital, Tana Toraja, Indonesia
4Otolaryngologist in Cut Meutia Hospital, North Aceh, Indonesia
Correspondence to: Kristanty Randa Arung, Psychiatrist in Lakipadada General Hospital, Tana Toraja, Indonesia.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
A vaccine program is one of the Indonesian government's measures to combat the covid-19 outbreak. The goal of this study is to see if there's a link between Adverse Events Following Immunization (AEFI) symptoms and anxiety levels after receiving the covid-19 vaccine. 100 respondents who worked at Lakipadada General Hospital and had received full doses (third dose) of covid-19 vaccinations were surveyed using a correlation analytic methodology with cross-sectional methodologies. AEFI Questionnaire which was developed and validated by INDOS ORL HNS (The Indonesian Society of Otorhinolaryngologist Head and Neck Surgeon), and the Beck Inventory Anxiety Questionnaire (BAI) were used to determine the level of anxiety among the participants. After respondents received the first dose of covid-19 vaccine (p= 0.041 and r = 0.154) and the third dose (p = 0.003 and r = 0.423), there was a significant correlation between adverse events following immunization symptoms and anxiety levels, but there was no meaningful correlation after administering the second dose vaccine (p = 0.315 and r = 0.077). Anxiety levels are minimum (23%), mild (74%), and moderate (3%). Shaky (p = 0.000, r = 1,000), weak / lightheaded (p = 0.012, r = 1,000), and unsteady (p = 0.000, r = 0.917) are all anxiety symptoms that show a substantial connection with adverse events following immunization. The first dosage of vaccine is sinovac (100%), the second dose is sinovac (99%) and astrazeneca (1%), and the third dose is moderna (99%) and astrazeneca (1%).
Keywords:
Adverse Events Following Immunization (AEFI)Symptoms, Anxiety and Covid-19 Vaccine
Cite this paper: Kristanty Randa Arung, Tjandra Manukbua, Triapermatasari Buntugayang, Indra Zachreini, Correlation of Adverse Events Following Immunization (AEFI) and Anxiety Levels After Covid-19 Vaccination, International Journal of Clinical Psychiatry, Vol. 9 No. 1, 2022, pp. 1-6. doi: 10.5923/j.ijcp.20220901.01.
1. Introduction
The covid-19 pandemic has had an impact on many elements of society, including employment, finance, health, and social life, all of which can have an impact on one's mental health [1]. In several countries around the world, some studies suggest an increase in psychological suffering, including anxiety and suicide ideation [2]. Another study found that economic troubles are most significantly linked to declining mental health, whereas health worries and social distance in society are also linked to it, albeit the link is weaker [3,4,5].Given the lengthy covid-19 pandemic and its substantial influence on the economy and social life, massive measures to combat coronavirus disease 2019 (covid-19) must be made with multiple strategies. Due to a lack of public understanding about the application of healthy measures, the danger of transmission is increasing. Intervention is required not only to implement health measures, but also to break the chain of covid-19 virus transmission through a vaccination program. The goal of the covid-19 vaccination program is to limit covid-19 virus transmission, reduce morbidity and mortality rates, achieve community immunity (herd immunity), and protect the public from covid-19 so that they may continue to work socially and economically [6,7,8,9].
2. Methods
2.1. Subjects
A correlation analytical research design with cross-sectional methodologies was employed in this study, which included 100 respondents who worked at Lakipadada Hospital. The following were among the requirements for inclusion: (a). The entire dose of covid-19 vaccination (third dose) was given to the subject (b). Between the ages of 20 and 55, (c). sex (male or female) and (d). I was able to connect to the internet. The research took place during August and October of 2021. The Lakipadada Ethics Committee gave their approval to the study.
2.2. Design and Procedure
A correlation analytical research design was used in this investigation. The Lakipadada Ethics Committee in Lakipadada Hospital in Tana Toraja, Indonesia, accepted it, and all experiments were carried out in conformity with relevant standards and regulations. All participants were given written informed consent to participate in this study and for the results to be published after a comprehensive explanation of the study protocols.The following are the study's procedures:1) Respondents completed the Adverse Events Following Immunization (AEFI) Questionnaire, which was issued and validated by the INDOS ORL HNS (The Indonesian Society of Otorhinolaryngologist Head and Neck Surgeon), as well as the Beck Inventory Anxiety (BAI) Questionnaire, both of which were completed online using Google Forms, access on https://forms.gl/ZsfZa5ASWfGHTpTcA for the Adverse Events Following Immunization (AEFI) form, and go to https://forms.gle/cogBXWQHxAGdmSYc7 for the Beck Inventory Anxiety Questionnaire (BAI) form.2) The data is analyzed using SPSS version 20 and the statistical method of the Correlation Of The Contingencies Coefficient and Lambda.
2.3. Measurement
2.3.1. Psychometric Measures
There are two instruments used in this study:§ The Beck Anxiety Inventory (BAI) was developed by Aaron T. Beck to assess the absence of symptoms as well as the intensity of anxiety in people. This questionnaire is a self-report questionnaire with 21 multiple-choice items. Each question is given a score ranging from 0 (not at all) to 3 (very severe), as well as the accompanying information: 0 means not at all, 1 means mild, 2 means moderate, and 3 means severe. The total value for each item is calculated by adding the values for each item, with the total value ranging from 0 to 63 points. The following is the interpretation value:- Total score 0 - 7: Minimum anxiety level- Score 8 – 15: Mild anxiety level- Score 16-25: Moderate anxiety levels - Score 26 - 63: Severe anxiety levelBAI’s scale validity and reliability test scores range from 0.92 to 0.94 (Alpha Cronbach) [11,12].§ The Adverse Events Following Immunization (AEFI) Questionnaire which has been issued and validated by the INDOS ORL HNS (The Indonesian Society of Otorhinolaryngologist Head and Neck Surgeon) which consisting of 24 questions.
2.3.2. Statistical Analysis
The data was processed and presented using the statistical program (SPSS) version 20. The data will be evaluated utilizing the correlation of contingencies coefficient and lambda statistical approach.
3. Results
3.1. Subjects characteristic
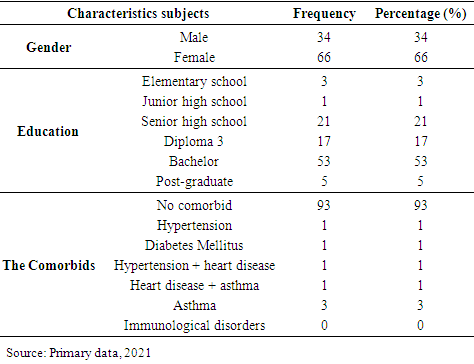
The demographic characteristics of the respondents are shown in table 1. Table 1. Demographics of respondents
 |
| |
|
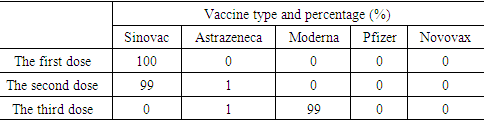
Based on the demographics subjects (table 1), most of the respondents are female (66%), with the highest level of education are bachelor (53%). The respondents who didn’t have comorbid diseases before given covid-19 vaccine are 93%. But there are also respondents who suffer with comorbid diseases such as asthma (3%), hypertension (1%), diabetes mellitus (1%), hypertension and heart disease (1%), heart disease and asthma (1%).Table 2. The characteristics respondents based on the type of covid-19 vaccine obtained
 |
| |
|
Based on table 2, all the respondent's first vaccine dose was sinovac (100%), the second vaccine dose consisted of sinovac (99%) and astrazeneca (1%), while the third dose they received moderna (99%) and astrazeneca (1%).Table 3. The characteristics based on how to get information related to the covid-19 vaccine
 |
| |
|
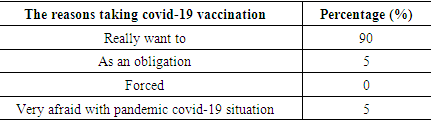
Table 4. The characteristics based on reasons taking covid-19 vaccination
 |
| |
|
Based on table 3 and 4, most of the respondents received information related to the covid-19 vaccine from mass media and discussions with friends (69%). The most reasons why they want to be vaccinated include: really want to (90%), as an obligation (5) and they was very afraid with pandemic covid-19 situation (5%). Table 5. The characteristics based on covid-19 survivor history
 |
| |
|
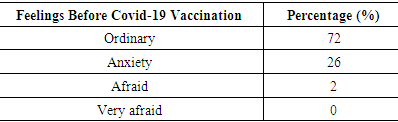
Table 6. The Characteristics based on feelings before obtaining covid-19 vaccination
 |
| |
|
Based on table 5 and 6, most of the respondents who received the covid-19 vaccine had not been exposed to the covid-19 virus (99%). The feelings before they getting the covid-19 vaccine varied widely, including: feeling ordinary (72%), feel anxiety (26%) and afraid (2%).
4. Statistic Analysis
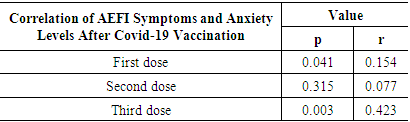
AEFI and the BAI Questionnaire were completed online after the respondents received the third dose of covid-19 immunization. The collected data is then examined using SPSS version 20 and the Contiguous and Lambda Coefficient Correlation Test statistical procedures.Table 7 shows that there was a significant link between AEFI symptoms and anxiety levels following the administration of the first dose of covid-19 vaccination and the third dosage, with a p 0.05 value and a weak (r = 0.154) to moderate (r = 0.423) correlation strength.Table 7. Correlation of AEFI Symptoms and Anxiety Levels After Covid-19 Vaccination
 |
| |
|
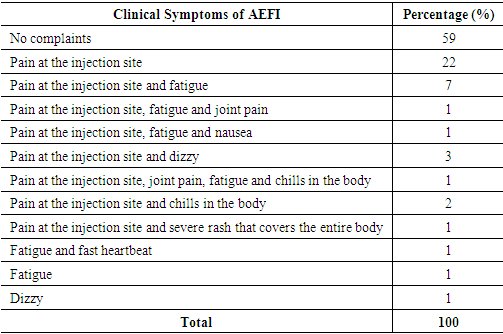
The following are the clinical signs of AEFI after the first dose of covid-19 vaccine:Table 8. Symptoms of AEFI after the first dose of Covid-19 Vaccine
 |
| |
|
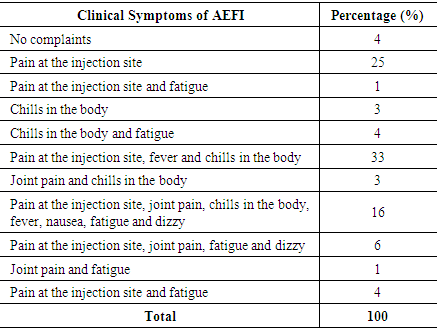
While the following are the clinical signs of AEFI after the third dose of covid-19 vaccine:Table 9. Symptoms of AEFI after the third doses od Covid-19 Vaccine
 |
| |
|
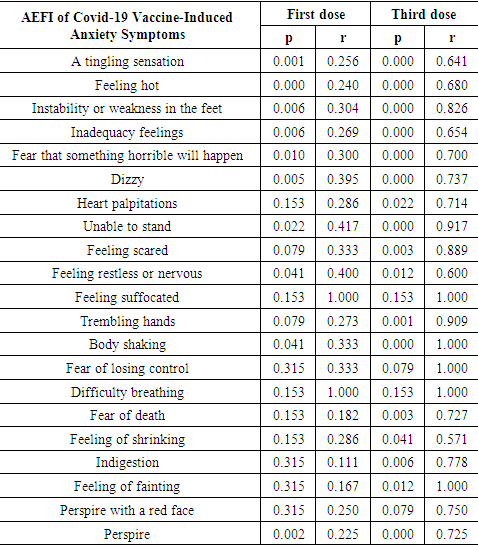
Table 10. After the third dose of Covid-19 vaccination, the anxiety symptoms based on BAI Questionnaire
 |
| |
|
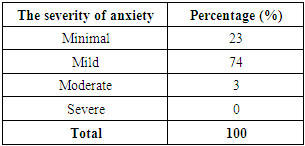
Table 11. The severity of anxiety caused by AEFI symptoms
 |
| |
|
Following the collection of data on AEFI symptoms caused by the administration of the first and third doses of covid-19 immunization, the investigation was extended to look at the link of anxiety symptoms caused by the AEFI symptoms.
5. Discussion
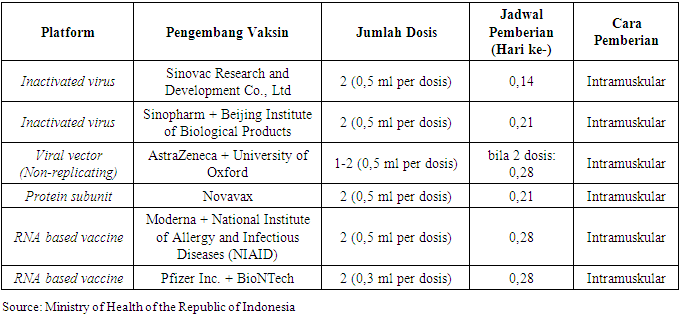
According to the study subjects characteristic data, 93% of respondents did not have comorbidities before to receiving the covid-19 vaccine (93%), implying that prior to receiving the covid-19 vaccine, respodenes were subjected to rigorous screening. If infected with the covid-19 virus, people with comorbid conditions are more likely to have severe symptoms. Diabetes mellitus, hypertension, cancer, cardiovascular disease (stroke and heart disease), kidney disease, chronic lung disease (such as COPD and asthma), liver disease, dementia, immune disorders (such as malnutrition and HIV), and autoimune disease are all diseases that can cause comorbidities in covid-19 patients (such as SLE and rheumatoid arthritis). People with comorbid conditions are one of the most vulnerable groups to covid-19 infection, thus they must be more cautious in taking efforts to prevent it, one of which is getting the covid-19 vaccine. Currently, the covid-19 vaccination can be given to patients with comorbid disorders, and it is regarded safe and effective as long as the disease is adequately controlled by medical care. Patients with comorbid diseases are vaccinated to prevent the onset of severe symptoms and fatal illnesses caused by infection with the covid-19 virus. However, in order to avoid and reduce the incidence of adverse effects that could jeopardize the patient's condition, the covid-19 vaccination must be administered with caution and with appropriate medical considerations. [14]Respondents received sinovac (100%) for the first dose, sinovac (99%) and astrazeneca (1%) for the second dose, and sinovac (99%) and astrazeneca (1%) for the third dose (1%).Table 12. Types of Covid-19 Vaccines in Indonesia
 |
| |
|
Covid-19 vaccinations are only provided by the government if they have been proven safe in clinical trials and have received an Emergency Use Authorization (EUA) from BPOM. [15]Almost all of the respondents (90%) are willing to engage in the government's covid-19 vaccination program, which was initiated in an effort to combat the pandemic. Respondents learned about covid-19 immunization from a variety of sources, including the news, conversations with acquaintances, and reading scientific literature. People who get notifications via Short Message Service (SMS) Blast must participate in the execution of the covid-19 vaccination, according to the government. [14]When it came to receiving covid-19 immunization, the majority of responders (72%) felt usual, but there were also those who felt worried (26%) and afraid (2%). Anxiety and fear occur as a result of concerns about the vaccine's safety and effectiveness, despite the government's efforts to educate the population to be willing to be vaccinated as a means of assisting the government in combating the Covid-19 pandemic. [15-16]After the first dose of vaccine administration (p = 0.04, r = 0.154) and the third dosage (p = 0.003, r = 0.423), there is a significant correlation between anxiety and the manifestation of symptoms of the covid-19 AEFI. Anxiety following the first dosage (100% sinovac vaccine) is a psychological reaction resulting from concerns about the safety and efficiency of the covid-19 vaccine, which has just recently been used worldwide. While there was no meaningful link in the second vaccine dose because virtually all of the respondents received the same type of vaccine as the first dose (99% sinovac), the respondent's self-adjustment mechanism was able to overcome the anxiety caused by AEFI in the second dose. In contrast to the first and second doses, almost all responses (99% of current vaccines) receive a new type of vaccine after the third dose, and the side effects of AEFI that occur after the third dose have more severe symptoms than AEFI symptoms in the first and second doses.AEFI is a medical occurrence that may be linked to vaccination. A vaccination reaction, a procedure error, a coincidence, an anxiety reaction, or an unclear causative association can all occur. The incidence of AEFI is regarded as serious if the medical event caused by each dose of vaccination administered results in death, hospitalization, or life-threatening sequelae. Because the vaccine used in the covid-19 vaccination program still contains a new vaccine, passive surveillance of AEFI and active surveillance of Follow-up Events with Special Attention are required to assess its safety (KIPK). [14,15]The responses that may occur after receiving the covid-19 vaccine are similar to those that may occur after receiving other vaccines. These are some of the symptoms: [14]1. Local responses such as: § Pain, redness, and swelling at the injection site,§ Cellulitis and other serious local responses.2. Systemic effects such as:§ Fever, § Myalgia (muscle pain all over the body),§ Atralgia (joint pain), § Weakness, § Headache.3. Other reactions, such as: § Allergic reactions such as urticaria, oedem, § Anaphylactic reaction, § Syncope (fainted).Health care workers may advise vaccine recipients to apply a cold compress to the injection site and take paracetamol medication at a dose if they experience local mild reactions such as pain, swelling, or redness. Health care personnel may advise vaccine recipients to drink more, wear comfortable clothing, compress or take a warm bath, and take paracetamol medication at recommended doses if they experience minor systemic responses like fever and malaise. [14,15]Due to the possibility of AEFI-related procedural errors, a vaccination service system comprised of competent implementing officers (who have sufficient knowledge, are skilled in administering vaccinations, and have a professional attitude as health workers), complete equipment, and clear technical instructions must be prepared to the greatest extent possible. The technical instructions must be understood by all levels of government involved in this system. [14,15]Any AEFI that has nothing to do with vaccines or coins should be avoided. As a result, the target health state to be vaccinated should be screened as thoroughly as feasible. [14,15]Minimal anxiety (23%), mild anxiety (74%), and moderate anxiety (73%) were the three levels of anxiety associated with kipi symptoms following covid-19 immunization (3 percent). Body shaking (p = 0.000, r = 1,000), fainting (p = 0.012, r = 1,000), and inability to stand (p = 0.000, r = 0.917) are all anxiety symptoms that have a substantial link with AEFI symptoms.According to the World Health Organization, immunization-related anxiety reactions (IARR) are a range of signs and symptoms that can occur as a result of worry and are not caused by vaccine products, vaccine flaws or damage, or vaccination program or process errors. Anxiety reactions that appear to be linked to vaccination include: 1) Increased heart rate, hyperventilation (rapid and deep breathing), dry mouth, perspiration, and tingling are all signs of an acute stress reaction.2) Symptoms of a vasovagal reaction include a decrease in heart rate, blood pressure, hyperventilation, visual difficulties, syncope (a brief period of fainting lasting less than 20 seconds), and moderate vertigo.3) Muscle weakness, even paralysis, aberrant limb motions, difficulty walking and speaking, and non-epileptic seizures are all symptoms of neurological dissociation reactions with or without seizures. [15,16]Several trigger variables are linked to the emergence of vaccination-related anxiety reactions:§ Internal Factors: adolescents and women are more prone to anxiety reactions, as are certain personality types, a history of anxiety or mental problems, a history of needle phobia, and a history of drug intake or substances that affect psychiatric diseases.§ External Factors: the quantity of disinformation and false information shared through social media, poor vaccine-related experiences, a lack of trust in health services, and health personnel' lack of understanding regarding the possibility of anxiety reactions to vaccination and how to address it. [16]
6. Conclusions
After respondents got the first and third doses of the covid-19 vaccine, there was a significant correlation between AEFI symptoms and anxiety levels, but no meaningful correlation was seen after the second dose. The level of anxiety that occurs is minimum (23%), mild (74%), and moderate (3%). Body shaking, fainting, and inability to stand are all anxiety symptoms that have a high association with AEFI symptoms.
ACKNOWLEDGEMENTS
The authors declare that there is no conflict of interest regarding the publication of this paper. The authors would like to acknowledge the important support and contributions of INDOS ORL HNS (The Indonesian Society of Otorhinolaryngologist Head and Neck Surgeon).
References
| [1] | Francisco PA, Angrisani M, Bennett D, Darlig J, Kapteyn A and Thomas K. Covid-19 vaccines and mental distress: A Research Article. Plus One, 2021; http:/doi.org/10.1371/journal.pone.025606. |
| [2] | Patil ST, Datar MC, Shetty JV, Naphade NM. Psychological consequences and coping strategies of patients undergoing treatment for covid-19 at a tertiary care hospital: A qualitative study. Asian J Soc Health Behav 2021; 4:62–8. |
| [3] | Pramukti I., Strong C., Sitthimongkol Y., Setiawan A., Pandin M. G. R., Yen C.-F., et al. Anxiety and suicidal thoughts during the covid-19 pandemic: A cross-country comparison among Indonesian, Taiwanese, and Thai university students. Journal of Medical Internet Research. 2020; 22(12), e24487. pmid: 33296867. |
| [4] | Ampfen F., Kohler I.V., Ciancio A., Bruine de Bruin W., Maurer J. and Kohler H.P.,. Predictors of mental health during the covid-19 pandemic in the US: Role of economic concerns, health worries and social distancing. PloS one. 2020; 15(11), p.e0241895. pmid: 33175894. |
| [5] | Alimoradi Z., Broström A., Tsang H. W. H., Griffiths M. D., Haghayegh S., Ohayon M. M., et al. Sleep problems during covid-19 pandemic and its’ association to psychological distress: A systematic review and meta-analysis. EClinical Medicine. 2021; 36, 100916. pmid: 34131640. |
| [6] | Ministry of Health of the Republic of Indonesia, Vaccine Status. https://vaksin.kemkes.go.id/#/vaccines. |
| [7] | Decree of the Minister of Health of the Republic of Indonesia Nomor HK.01.07 / Menkes /12758 / 2020 About The Determination of Vaccine Types for the Implementation of Corona Virus Disease 2019 (Covid-19) Vaccination. |
| [8] | Decree of the Minister of Health of the Republic of Indonesia Number HK.01.07 / Menkes / 4638 / 2021 About Technical Instructions for The Implementation of Vaccination in the Context of Countering the Corona Virus Disease 2019 (Covid-19) Pandemic. 2021. |
| [9] | Regulation of the Minister of Health of the Republic of Indonesia Nomor 19 tahun 2021 about the second Amendment to the Regulation of the Minister of Health Number 10 of 2021 concerning the Implementation of Vaccination in the Context of Countering the Corona Virus Disease 2019 (Covid-19) Pandemic. 2021. |
| [10] | Hause A, Gee J, Johnson T, Jazwa A, et all. Anxiety-Related Adverse event Clusters After Janssen Covid-19 Vaccination-Five U.S. Mass Vaccination Sites; A Morbidity and Mortality weekly Report, 2021; Vol. 70/ No.18. |
| [11] | Beck Anxiety Inventory (A Complete guide). Written by PsychReel Medically Reviewed by our scientific review board. Page last updated: June 20, 2021. Next Review date: 20/06/2023. |
| [12] | Beck Anxiety Inventory Author/Affiliation Michael M. Grant, PhD Coastal Center for Cognitive Therapy, PA 1101 Johnson Avenue, Suite 200 Myrtle Beach, SC 29577 843.839.9028 www.coastalcognitive.com. |
| [13] | Provision of Covid-19 Vaccination in patients with concomitant / comorbid diseases in PAPDI Recommendations, The Association of Internal Medicine Specialists in Indonesia (PAPDI), March 18, 2021. |
| [14] | Technical Instructions for the Implementation of Vasination in the Figures for The Prevention of Corona Virus Disease 2019 (Covid-19) in the Decree of the Minister of Health of the Republic of Indonesia Number HK. 01.07 / MENKES / 4638 / 2021. |
| [15] | About the Implementation of Covid-19 Vaccination in Frequently Asked Question (FAQ) Ministry of Health of the Republic of Indonesia, 2021. |
| [16] | Silmi ZI. KIPI or Vaccination-Related Anxiety Reactions? in COVID-19 Control, KawalCOVID19.id, April 19, 2021. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML










