Shweta Chauhan, Seema Singh Parmar
Department of Psychiatry, Teerthanker Mahaveer University, Moradabad, India
Correspondence to: Shweta Chauhan, Department of Psychiatry, Teerthanker Mahaveer University, Moradabad, India.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Aims and Objectives: To assess and compare the onset of antidepressant actions of esitalopram, fluoxetine, sertraline and paroxetine with vilazodone. Methodology: One hundred and fifty patients diagnosed with Depression according to the DSM 5 criteria, seen in the Out-patient department of psychiatry at a tertiary care hospital, participated in the study after obtaining written and informed consent. Thirty patients each were randomly assigned to treatment with either of the following drugs: esitalopram, fluoxetine, sertraline, paroxetine and vilazodone. Montgomery Asberg Depression Rating Scale (MADRS) and Hamilton Depression Rating Scale (HAM-D) were applied on Day 1 to assess the baseline severity of Depressive features and again at week 1, week 2 and week 8 following initiation of treatment, to assess the extent of improvement, if any, along with onset of antidepressant action with various drugs. Results were tabulated using SPSS v20 and independent sample t tests were applied. Results: While all the drugs showed a decrease in depressive symptoms, Vilazodone was associated with significant differences between the day 1 and week 1 values of both MADRS and HAM-D scores as compared to other antidepressants in the study. Conclusion: The relatively faster onset of antidepressant action associated with Vilazodone can be useful in treating severe depression especially in those associated with suicidal tendencies and can thus be useful in achieving response and remission in patients suffering from Depression.
Keywords:
Vilazodone, Depression, MADRS, HAM-D
Cite this paper: Shweta Chauhan, Seema Singh Parmar, Is Vilazodone Really the Answer to the Delay Associated with the Onset of Antidepressant Action of SSRIs? – A Randomised Control Trial, International Journal of Clinical Psychiatry, Vol. 6 No. 1, 2018, pp. 9-18. doi: 10.5923/j.ijcp.20180601.02.
1. Introduction
According to the recent WHO 2017 statistics [1], approximately 300 million people suffer from Depression worldwide with females affected twice more commonly as compared to males. Prevalence of Depression varies worldwide – as high as 17% in the US to 9% in India [2]. The point prevalence of Depression in general out patients has been estimated to be 10%. The disability due to Depression has risen steadily with 2010 Global Burden of Disease study assigning it as the leading cause of disability, next only to HIV-AIDS [3]. This has prompted the World Health Organization (WHO) to include depression in the priority conditions listed in its Mental Health GAP action programme [4] (mhGAP) which aims to reduce the burden of selected mental illnesses.The first antidepressants were Mono Amine Reuptake Inhibitors and Tricyclic Antidepressants, however the advent of Selective Serotonin Reuptake Inhibitor (SSRI) revolutionized the treatment of Depression and till date is the greatest discovery in the treatment of Depression. The introduction of SSRI markedly reduced suicide rates in both adults and adolescents, but they were not entirely free of burdensome side effects like increased sleep, gastric discomfort in the early stages and sexual adverse effects to name a few.But the main drawback of SSRI agents is their delayed onset of action. An average of 2 weeks delay in the start of treatment with antidepressant agents and onset of clinical antidepressant action has been seen with almost all SSRIs, which forms the main setback of their clinical profile. This delay in the onset of action has been attributed to the time taken for the downregulation of somatodendritic 5HT1a receptors. With a view of developing a single drug which combines both the actions of 5HT1a agonism and SERT (Serotonin Transporter) antagonism, the molecule Vilazodone was developed (2011). Vilazodone shows partial agonism at the 5HT1a receptors which is why it is classified as Serotonin Partial Agonist Reuptake Inhibitor (SPARI) [5].Studies conducted so far have been placebo control trials, comparing the efficacy of Vilazodone to placebo in the treatment of depression. This study aims to compare the antidepressant effect of Vilazodone with four of the SSRI antidepressant drugs – Esitalopram, Sertraline, Paroxetine and Fluoxetine with emphasis on the time taken for the onset of antidepressant action.
2. Methodology
The study was conducted on one hundred and fifty patients who visited the Outpatient department of Psychiatry at a tertiary care hospital located in western Uttar Pradesh which caters to a primarily rural and sub-urban population after obtaining approval form the Institute’s Ethics Committee. Patients were diagnosed as suffering from depression on the basis of diagnostic criteria laid down by DSM-5, and only those who fulfilled these diagnostic criteria were included in the study. Patients were equally and randomly divided into five groups of thirty each to receive either of the five drugs: vilazodone, esitalopram, paroxetine, fluoxetine or sertraline. Patients were randomly assigned to each class after randomisation using paper chit method. Baseline values were noted on MADRS and HAM-D scales (day 1 scores) after which treatment with one of the above mentioned antidepressants was started. Follow up MADRS and HAM-D scores were taken after week 1, week 2 and lastly after week 8 of starting treatment. At the follow ups MADRS and HAM-D were administered to assess the efficacy and the relative onset of antidepressant actions of each drug.The data was collected and entered in MS Excel. Data was analysed using SPSS 20.0 and statistical differences in proportion were calculated using the chi square test, t-test and ANOVA.The results were explained in the form of baseline identification and socioeconomic parameters of the subjects in all five drug groups (vilazodone, esitalopram, paroxetine, fluoxetine and sertraline) in terms of descriptive and inferential statistics. Descriptive statistics were explained by frequencies, mean, standard deviation and range for numeric variables and by proportions and percentages for categorical variables in (vilazodone, esitalopram, paroxetine, fluoxetine and sertraline) groups. Inferential statistics were done by applying statistical test for significance testing by t test for continuous variables, univariate analysis wherever applicable and with 95% confidence intervals and Chi-square test for proportions and categorical variables. Following the derivation of initial results, post hoc analysis was done using Dunnett’s Test. In post hoc analysis, vilazodone was taken as the control (J drug) and the other four drugs (I drug) were compared against it.
3. Results
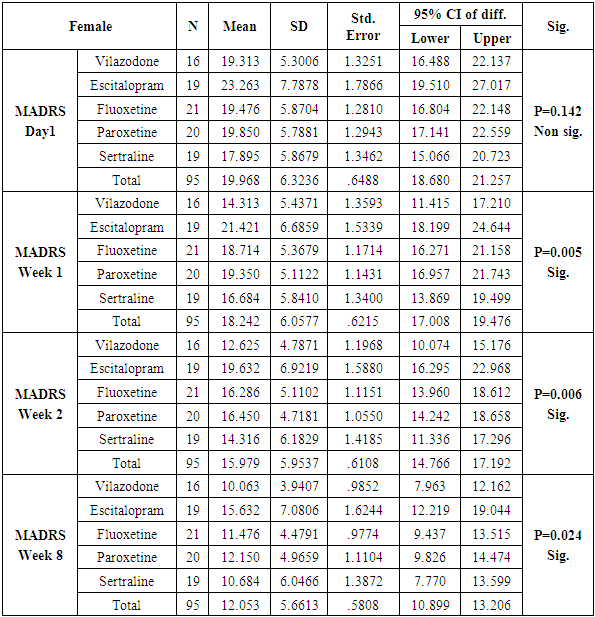
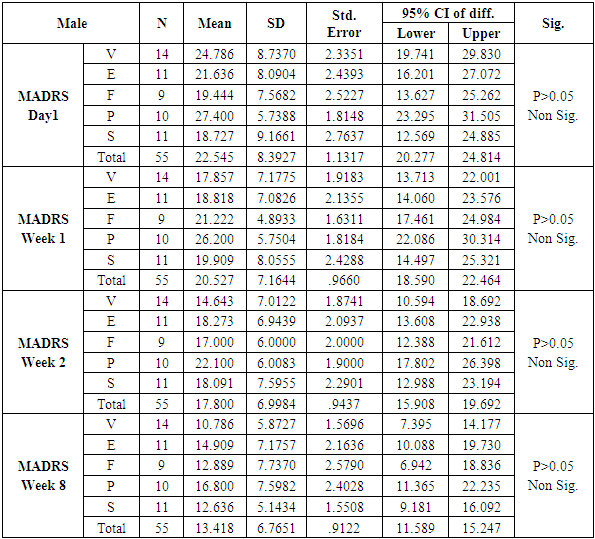
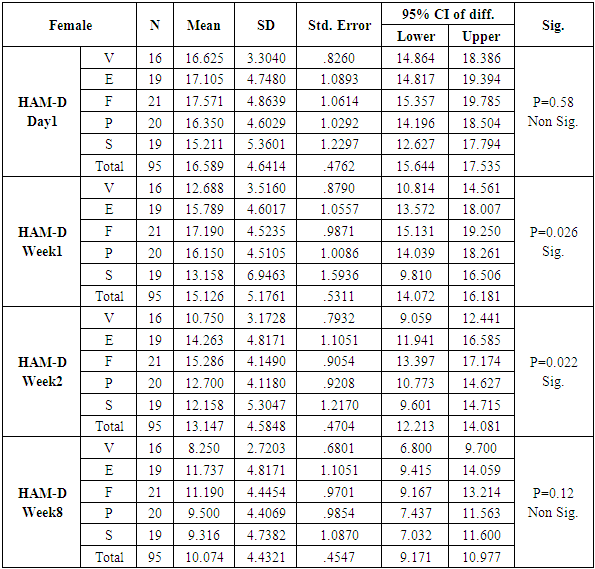
The number of females was almost twice the number of males amongst the sample size of the study (females N=95 and males N=55). In this study no specific age group showed significantly higher rates of depression as compared to other age groups. Many sociodemographic factors such as unemployment, lack of social and familial support have also been positively linked to depression [6]. In this study no significant association between the occurrence of depression and various socio demographic factors was noted.The study revealed that there was no significant difference in the treatment of depression with either of the five drugs used in the study (Tables 1, 2, 3, 4). Mean HAM-D and MADRS scores reductions were comparable in all five drug groups (Charts 1, 2, 3, 4). The findings that Vilazodone has a relatively rapid onset of action was seen in this study when it was compared directly with esitalopram, fluoxetine, paroxetine and sertraline. There was a significant reduction in week 1 values of MADRS scores in patients belonging to the vilazodone group as compared to the other groups (Tables 5, 6, 7, 8). These findings were also replicated on the HAM-D scores at week 1.Table 1. Table showing descriptive statistics of MADRS scores and test of significance for mean difference across all test drugs at various time intervals
 |
| |
|
Table 2. Table showing descriptive statistics of MADRS scores and test of significance for mean difference across all test drugs at various time intervals
 |
| |
|
Table 3. Table showing descriptive statistics and test of significance for mean of HAM-D scores across all test drugs at various time intervals
 |
| |
|
Table 4. Table showing descriptive statistics of HAM - D scores and test of significance for mean difference across all test drugs at various time intervals
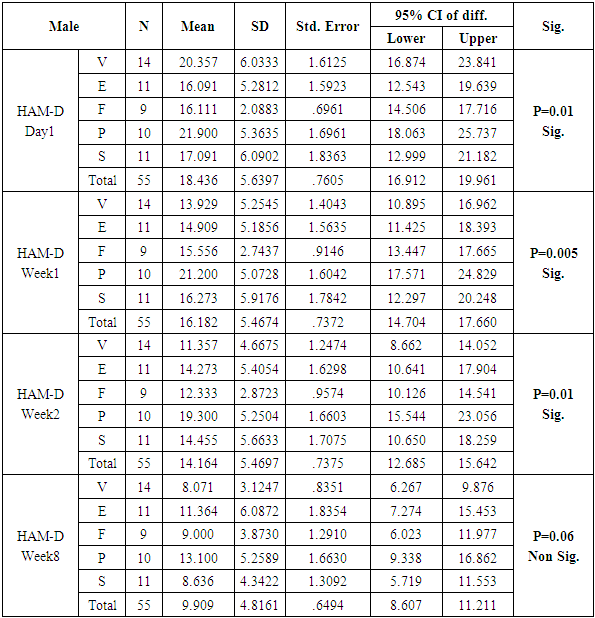 |
| |
|
Table 5. Descriptive statistics and Test of significance of mean MADRS Score between Vilazodone and Escitalopram at various time intervals
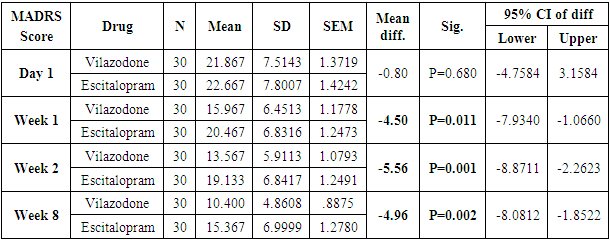 |
| |
|
Table 6. Descriptive statistics and Test of significance of mean MADRS Score between Vilazodone and Fluoxetine at various time intervals
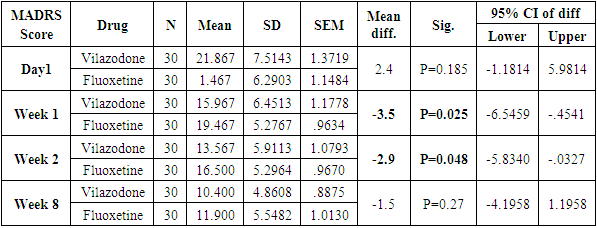 |
| |
|
Table 7. Descriptive statistics and Test of significance of mean MADRS Score between Vilazodone and Paroxetine at various time intervals
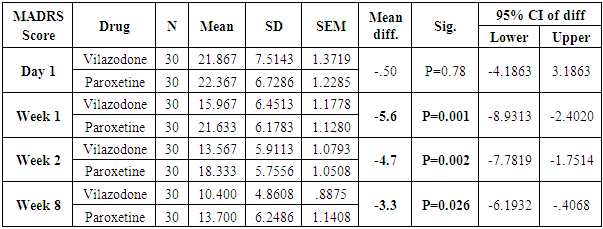 |
| |
|
Table 8. Descriptive statistics and Test of significance of mean MADRS Score between Vilazodone and Sertraline at various time intervals
 |
| |
|
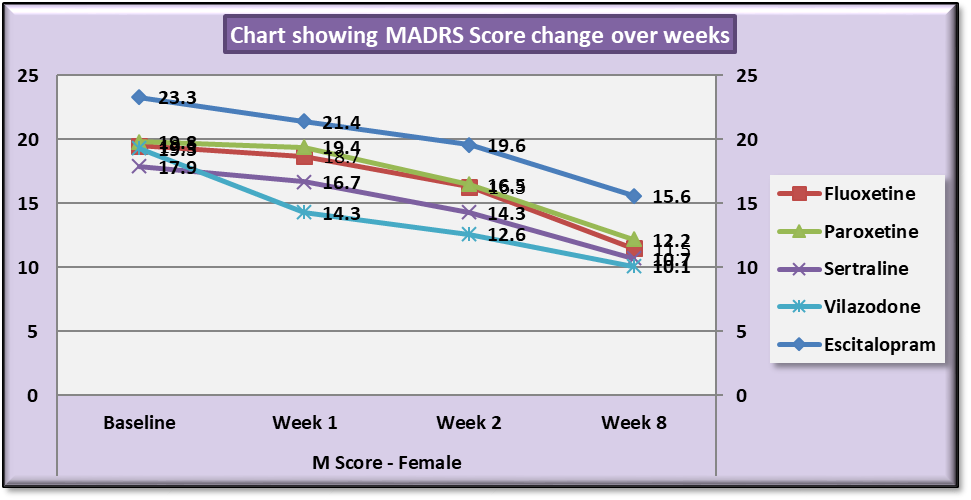 | Chart 1. Line Graph showing changes in mean MADRS score during the course of the study in females |
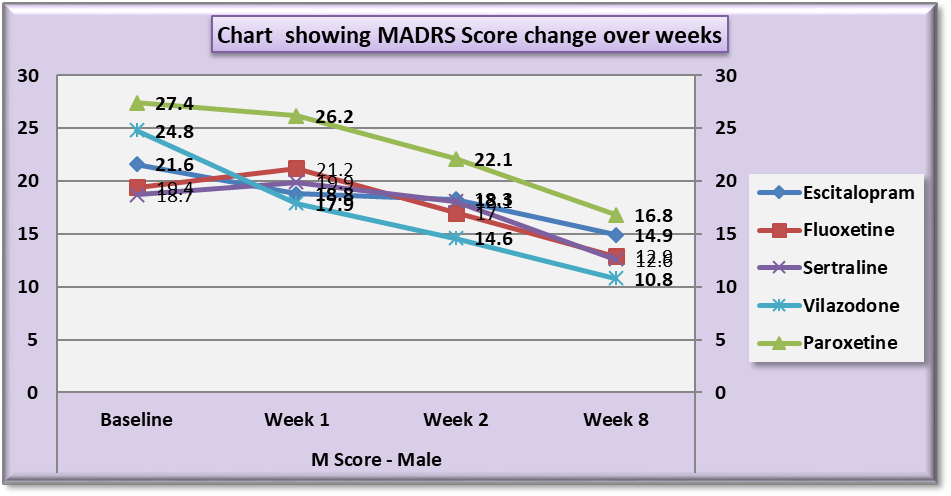 | Chart 2. Line Graph showing changes in mean MADRS score during the course of the study in males |
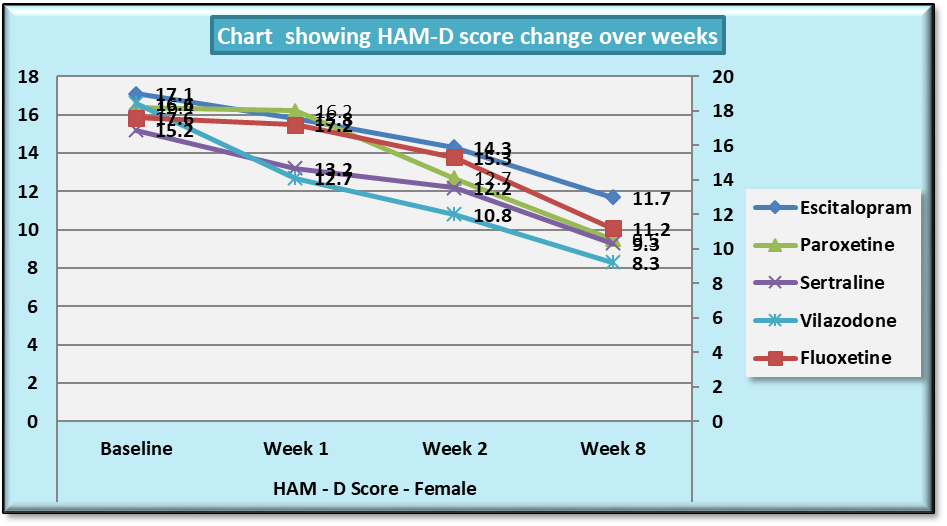 | Chart 3. Line Graph showing changes in mean HAM-D score during the course of the study in females |
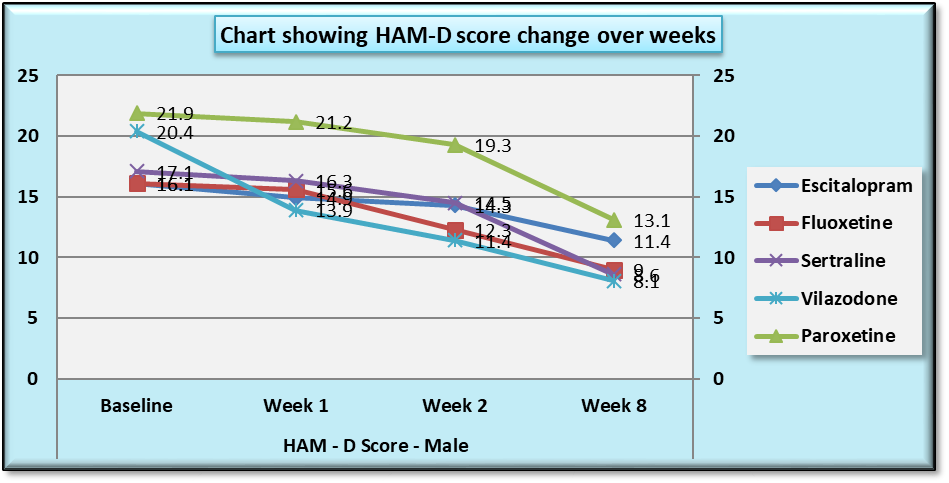 | Chart 4. Line Graph showing changes in mean HAM-D score during the course of the study in males |
Analysis also revealed that reductions in MADRS scores in females were more significant in all treatment groups as compared to males. Amongst females, esitalopram showed significant minimal reductions in week 1, week 2 and week 8 MADRS scores (p=.002, p=0.001 and p=0.012 respectively) (Table 9). Amongst males, paroxetine showed significantly minimal reductions in MADRS scores at week 1 and week 2 as compared to vilazodone (p=0.17 and p=0.38 respectively) (Table 10). While comparing the onset of antidepressant action on the basis of changes in week1 and week 2 MADRS scores, sertraline was most comparable to vilazodone.Table 9. Table showing results of Dunnett’s Test applied on MADRS scores across all test drugs at various time intervals in females
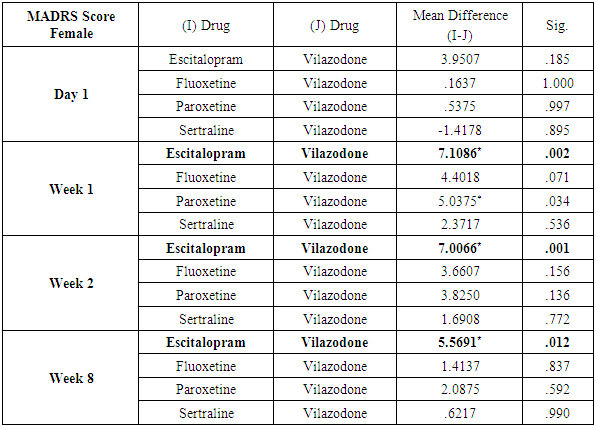 |
| |
|
Table 10. Table showing results of Dunnett’s Test applied on MADRS scores across all test drugs at various time intervals in males
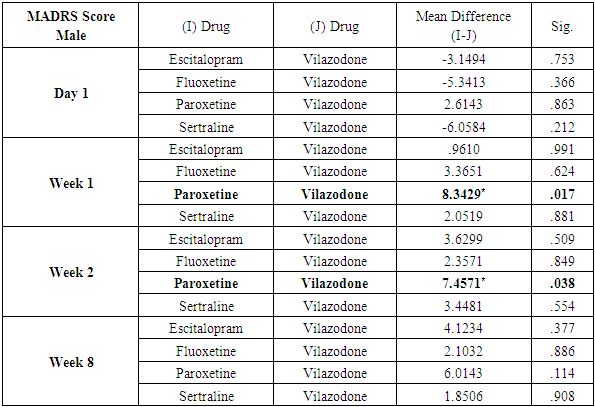 |
| |
|
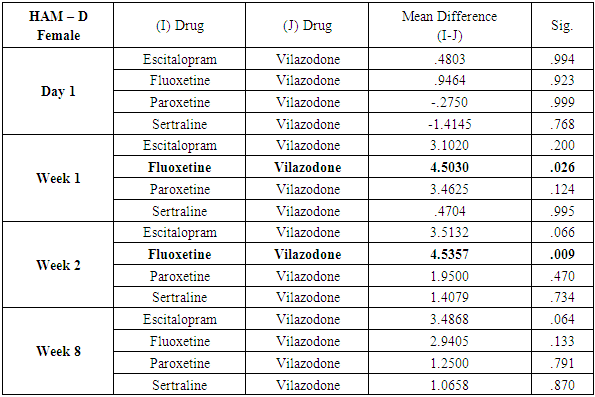
While similar findings were duplicated in the score reductions of HAM-D in males, paroxetine showed significantly less reductions at week 1, week 2 and week 8 (p=0.004, p=0.001 and p=0.039 respectively) (Table 11), results of HAM-D post-hoc analysis differed in females with fluoxetine showing most significant difference as compared to vilazodone (p=0.026 at week 1 and p=0.009 at week 2) (Table 12).Table 11. Table showing results of Dunnett’s Test applied on HAM – D scores across all test drugs at various time intervals in males
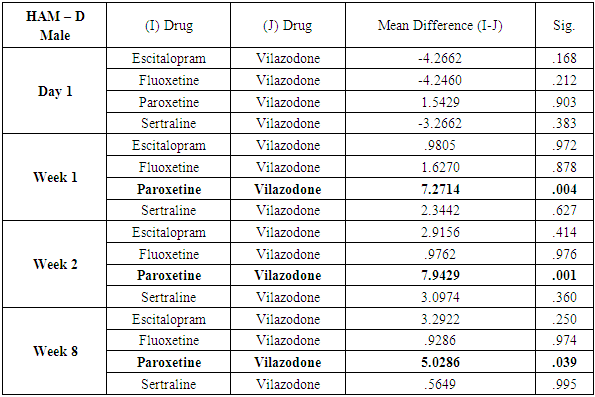 |
| |
|
Table 12. Table showing results of Dunnett’s Test applied on HAM – D scores across all test drugs at various time intervals in females
 |
| |
|
4. Discussion
The classical treatment of depression has involved the initiation of treatment with a single antidepressant agent, and either switching or augmenting the existing regimen only if no response is observed after a period of 4-6 weeks of observation. But evidences started emerging in the mid to late 2000s that initiating the antidepressant regimen with two drugs right from the beginning was seen to be associated with better response and lower remission rates [7]. This led to particular exploration of drugs which had more than one mechanism of action against depression in their pharmacological profile. Vilazodone is the result of such further experimentations as it combines the classical SERT inhibition with partial agonism at the 5HT1a receptors, which is seen in many atypical antipsychotics as well as the anxiolytic Buspirone.The first randomised trials which showed the efficacy of Vilazodone in patients with depression were conducted by Rickels et al in 2009 and Khan et al in 2011. Both these trials demonstrated superior efficacy of Vilazodone against placebo in patients with depression [8, 9]. This prompted further long term trials to study the efficacy and tolerability of Vilazodone in depression.Robinson et al (2011) [10] conducted a multicentric study at 52 different centres in the US on patients with MDD. The study showed that clinical improvement in depressive symptoms was seen across MADRS, CGI-S (Clinical Global Impressions - Severity) and CGI-I (Clinical Global Impressions - Illness), with a maximal change after 8 weeks of treatment which continued throughout the year.Khan et al (2014) [11] published a report on analysing the effectiveness of Vilazodone against the different symptoms of depression. They reported that the mean MADRS score of the patients at the start of treatment was 31.4. The mean MADRS score at the end of 8 week treatment was 21.1 (p<0.0001). Statistically significant improvement was seen as early as week 1 after initiating treatment with Vilazodone (p<0.01). According to a placebo controlled trial conducted by Croft et al (2014) [12], primary efficacy outcomes of Vilazodone using MADRS and CGI-S were significantly better than those in the placebo group (p<0.00001, effect size=0.54). While many such other studies have shown similar results, such as Citrome et al [13], McCormack in 2015 [14], most of the available data compares Vilazodone with placebo in patients suffering from Depression. Rele et al (2015) [15] conducted a double blind randomised trial on 60 subjects suffering from DSM-IV-TR MDD (Major Depressive Disorder). Patients receiving treatment with other antidepressants - fluoxetine, Esitalopram, citalopram, paroxetine, sertraline or venlafaxine – were randomised to three groups of varying doses of Vilazodone. All three arms of the study reported significant reductions in mean MADRS, CGI-S, CGI-I and HAM-D scores despite differing dosing schedules.Vilazodone has been directly compared with another antidepressant (paroxetine) in only one known study conducted by Eyre et al [16]. In this study both Vilazodone and Paroxetine showed significant decrease in HAM-D scores, however no significant differences were observed between the two groups.
5. Conclusions
To our knowledge, this was the first study to directly compare Vilazodone with other popular SSRI drugs as opposed to the placebo control trials. Whilst our study has shown similar results as the work which has been previously done on the subject, our study also attempted to evaluate the lag of onset of antidepressant action associated with SSRIs. This lag of onset can be very unsettling in patients struggling with severe depression or in those with suicidal ideation. Thus as per the results of this study, Vilazodone can be effectively used as a first line antidepressant in these patients. However similar long term longitudinal trials are required in this area for a better understanding of the drug and its unique mechanism of action.
References
| [1] | http://www.who.int/mediacentre/factsheets/fs369/en/. |
| [2] | Andrade L, Caraveo-anduaga JJ, Berglund P, Bijl RV, Graaf RD, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N. The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. International journal of methods in psychiatric research. 2003 Feb 1; 12(1): 3-21. |
| [3] | Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS medicine. 2013 Nov 5; 10(11): e1001547. |
| [4] | World Health Organization. mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings: mental health Gap Action Programme (mhGAP). Geneva: World Health Organization; 2010. |
| [5] | Schwartz TL, Siddiqui UA, Stahl SM. Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor. Therapeutic advances in psychopharmacology. 2011 Jun; 1(3): 81-7. |
| [6] | McKee-Ryan F, Song Z, Wanberg CR, Kinicki AJ. Psychological and physical well-being during unemployment: a meta-analytic study. Journal of applied psychology. 2005 Jan; 90(1): 53. |
| [7] | Stahl S. Combining antidepressant therapies from the initiation of treatment: a paradigm shift for major depression. J Clin Psychiatry. 2009; 70(11): 1493-1494. |
| [8] | Rickels K, Athanasiou M, Robinson DS, Gibertini M, Whalen H, Reed CR. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. The Journal of clinical psychiatry. 2009 Mar; 70(3): 326-33. |
| [9] | Khan A, Cutler AJ, Kajdasz DK, Gallipoli S, Athanasiou M, Robinson DS, Whalen H, Reed CR. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. The Journal of clinical psychiatry. 2011 Apr; 72(4): 441-7. |
| [10] | Robinson DS, Daniel KK et al, a 1-year open label study assessing the safety and tolerability of Vilazodone in patients with major depressive disorder. J Clin Psychopharmacol 2011; 31: 643-646. |
| [11] | Khan A, Sambunaris A, Edwards J, Ruth A, Robinson DS. Vilazodone in the treatment of major depressive disorder: efficacy across symptoms and severity of depression. International clinical psychopharmacology. 2014 Mar; 29(2): 86. |
| [12] | Croft HA, Pomara N, Gommoll C, Chen D, Nunez R, Mathews M. Efficacy and safety of vilazodone in major depressive disorder: a randomized, double-blind, placebo-controlled trial. The Journal of clinical psychiatry. 2014 Nov 24; 75(11): 1291-8. |
| [13] | Leslie Citrome, Gommoll CP, Xiongwen T et al. evaluating the efficacy of Vilazodone in achieving remission in patients with major depressive disorder. Int Clin Psychopharmacol 2015, 30: 75-81. |
| [14] | McCormack PL. Vilazodone: a review in major depressive disorder in adults. Drugs. 2015 Nov 1; 75(16): 1915-23. |
| [15] | Rele S, Millet R, Kim S, Paik JW, Kim S, Masand PS, Patkar AA. An 8-week randomized, double-blind trial comparing efficacy, safety, and tolerability of 3 vilazodone dose-initiation strategies following switch from SSRIs and SNRIs in major depressive disorder. The primary care companion for CNS disorders. 2015; 17(4). |
| [16] | Eyre H, Siddarth P, Cyr N, Yang H, Cole S, Forbes M, Lavretsky H. Comparing the Immune-Genomic Effects of Vilazodone and Paroxetine in Late-Life Depression: A Pilot Study. Pharmacopsychiatry. 2017 Apr 25. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML










