-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Clinical Psychiatry
p-ISSN: 2332-8355 e-ISSN: 2332-8371
2017; 5(3): 51-56
doi:10.5923/j.ijcp.20170503.01

The Correlation between Metamphetamine Abuse and Salivary Alpha Amylase (sAA)
Andi Jayalangkara Tanra, Saidah Syamsuddin, Sonny Teddy Lisal, Grace Catherine
Department of Psychiatry, Medical Faculty, University of Hasanuddin, Makassar, Indonesia
Correspondence to: Sonny Teddy Lisal, Grace Catherine, Department of Psychiatry, Medical Faculty, University of Hasanuddin, Makassar, Indonesia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Amphetamine like substance, including methamphetamine (METH) is the second most widely misused drug after cannabis in the world. Many evidences suggest that norepinephrine plays an important role in the effects of methamphetamine. Recently, the measurement salivary alpha-amylase (sAA) has been considered to be a useful tool for evaluating the symphatetic adrenal medullary (SAM) system. Salivary AA had not been extensively studied in metamphetamine abuse patients. The aim of this study was to determined the correlation between the level of methamphetamine abuse and the level of salivary alpha-amylase. This study was an observational analytic study with cross sectional design, conducted at Rehabilitation Center of National Narcotic Board in Baddoka and Provincial Narcotic Board of South Sulawesi, Indonesia. The population of the study was 40 methamphetamine abusers and 40 healthy control subjects. The level of methamphetamine abuse was assessed with WHO ASSIST V3.0 instrument. We used a portable and rapid hand-held monitor to measure sAA level. Salivary AA levels in metamphetamine abusers were significantly higher than in the control subjects (p=0.001). But there was no significant correlation between level of methamphetamine abuse and the level of salivary alpha-amylase (p>0.05, r=0.208). There were no difference in sAA levels among low, moderate and high risk metamphetamine abusers.
Keywords: Metamphetamine, Salivary alpha-amylase (sAA), Symphatetic adrenal medullary system (SAM), Norepinephrine
Cite this paper: Andi Jayalangkara Tanra, Saidah Syamsuddin, Sonny Teddy Lisal, Grace Catherine, The Correlation between Metamphetamine Abuse and Salivary Alpha Amylase (sAA), International Journal of Clinical Psychiatry, Vol. 5 No. 3, 2017, pp. 51-56. doi: 10.5923/j.ijcp.20170503.01.
Article Outline
1. Introduction
- The World Health Organization (WHO) has identified the use of alcohol, tobacco, and illicit drugs as one of the 20 highest risk factors for disease [1]. In 2011, the United Nations Office on Drugs and Crime (UNODC) estimated that between 167 and 315 million or 3.6% to 6.9% of the world's population aged 15-64 years use narcotics at least once a year [2].Amphetamine like substance, including methamphetamine (METH) is the second most widely misused drug type after cannabis in the world. The making, trading and use of methamphetamine has been a primary drug threat in Indonesia. The cases of METH abuse have increased in the last five years from 2009 to 2012. The confiscated results of Amphetamine Type Stimulants (ATS) in 2011 increased by 66% or 123 tons compared in 2010 that was 74 tons. From 3.7 to 4.7 million users of substances in Indonesia in 2011, about 1.2 million used methamphetamine [3]. Annual report conducted by Rehabilitation Center of National Narcotic Board Baddoka in 2015 and 2016, the most abuse drug type was methamphetamine [4, 5].Methamphetamine abuse is associated with a high rate of relapse. Brecht and Herbeck (2014) conducted the study of methamphetamine and found that 61% of methamphetamine abusers had relaps, a year after rehabilitation and only 13% of individuals remained abstinent from methamphetamine during 5 years of research. There is an urgent need to improve treatment options for METH abusers, since current psychological and pharmacological therapies for drug addicts are limited success [6].There are two types of stress, psychological and biological stress. Biologically, stress is a factor that disrupts homeostasis. Substance abuse is one of the biological stressors. Substance abuse activates both of Hipothalamus Pituitary Adrenal (HPA) and Symphatetic Adrenal Medullary (SAM system. Acute substance abuse, as well as acute psychological stress, activates the HPA system by increasing glucocorticoids (cortisol) and activates SAM system by increasing norepinephrine (NE). The effects of recurrent psychological stress on two major stress systems, as well as subsequent effects on motivational and behavioural or emotional control mechanisms. These effects are similar to effects induced by repeated substance use. Noradrenergic response (SAM) is sensitized or become more reactive due to chronic activation of the locus coeruleus. Chronic substance abuse increases risk for dependence, attenuate motivation to quit and increases relapse risk potentially by intensified sensitization of motivational systems, by a shift from positive to negative reinforcement due to sensitization of the amygdala by corticotropin releasing factor (CRF) and by increased sensitization of noradrenergic system [7].From previous study, it has been widely known that psychostimulants such as methamphetamine induce significant impairment of the function of the HPA. Repeated or chronic HPA system activation by repeated substance use can lead to impaired stress response, including blunted (impaired HPA system activation), prolonged responses (impaired HPA system inhibition) and a lack of HPA system habituation to repetition of the same stressor [7]. Alteration in the function of HPA are assosited to stress-related behaviors that contribute to addictive processes such as relapse. Several human studies have suggested that the function of the HPA system is disrupted by the recurrent use of metamphetamine. Impairment of stress hormone level in withdrawal phase contribute to the appearance of depressive and anxious symptoms [8, 9]. Drugs that relieve dysfunction of HPA system such as metyrapone and drugs that interact with CRF receptors and glucocorticoids offer an alternative mechanism for addiction therapy [10].Metamphetamine rapidly increases central dopamine and NE neurotransmission by acting as a substrate for presynaptic and vesicular monoamine transporters, reversing their action and increasing cystolic and synaptic transmitter levels [11]. Accumulating evidence indicates NE may play an important role in METH’s effects [12]. In fact, methamphetamine more potent induces the release of NE than dopamine [13, 14].Salivary alpha amylase is known as an enzyme responsible for the digestion process of carbohydrates and considered reflect changes in the autonomic nervous system. The release of sAA is caused by activation of the autonomic nervous system that controls the salivarious glands. Recently salivary alpha-mylase (sAA) has been studied as a biological marker of the sympathetic nervous system so it can be used to evaluate the activity of the SAM system [15]. The release of stress-induced plasma norepinephrine correlates with sAA activity [16]. Adrenergic agonist drugs and drugs that facilitate NE release also increase sAA activity, and otherwise adrenergic antagonists decrease sAA activity [17, 18].A previous study conducted by Haile et al (2013), to assessed the effect of methamphetamine on noradrenergic activity by measuring the levels of the sAA, showed that methamphetamine increased levels of the sAA [19]. Not much is known about the effect of methamphetamine abuse on the dysregulation of SAM system. Knowing the effect of METH on the SAM system, through measuring the sAA levels as an indicator for assessing SAM activity can lead to further research to achieve a more effective therapeutic strategy for drug addiction. Through this study the authors want to determine the correlation between level of methamphetamine abuse with sAA level, as an indicator to assess SAM system.
2. Methods and Material
- The study was an observational analytic study with cross sectional design.
2.1. Subjects
- The study subjects consisted of metamphetamine abusers who came to Rehabilitation Center of National Narcotics Board in Baddoka and Provincial Narcotic Board of South Sulawesi, Indonesia for rehabilitation therapy and healthy control subjects who were recruited between May and June 2017. Fourty metamphetamine abusers between 17 and 45 years old, who met the fifth-edition Diagnostic and Statistical Manual of Mental Disorders (DSM-V) criteria for stimulant (metamphetamine) related disorder, had a positive result for metamphetamine from urine test, and were able to provide sufficient information were selected to participate. Subjects were excluded if criteria were met for dependence on other drugs except for nicotine (smoking). Most subjects in metamphetamine group had used alcohol in the past but did not meet criteria for dependence. Control group subjects composed of 40 healthy volunteers recruited from staffs at Rehabilitation Center of National Narcotics Board in Baddoka, doctors and internship medical students in psychiatry department Hasanuddin University. Both groups did not showed any neurological and cardiovascular disease that could cause autonomic dysfunction, chronic metabolic diseases such as endocrine, kidney and liver diseases, and diseases in the mouth area. None of the subjects were taking any medication such as adrenergic agonist or antagonist drugs.All subjects were given informed consent following a description of the procedure, and all subjects were given opportunity to ask questions about the study. The study protocol was approved by the Ethics Committee of the Medical Faculty Hasanuddin University.
2.2. Procedure
- All subjects who met the inclusion criteria were interviewed to obtained the sample identity data including initials of name, gender, age, last education, occupation and marital status. Clinical interview conducted by researchers based on World Health Organization Alcohol, Smoking and Substance Involvement Screening Test Version 3.0 (WHO ASSIST V3.0) to determine methamphetamine abuse level (ASSIST risk score for metamphetamine). In this study we focused on specific substance involvement score for metamphetamine use. All subjects examined using a hand-held monitor (Nipro Co., Japan) to measure the sAA activity using reagent paper [20, 21]. Salivary AA was measured in the morning (10:00 - 14:00 pm), as the sAA activity shows a diurnal pattern with a steady increase in activity during the course of the day [22]. We did not investigate sAA activity at multiple time points. Saliva was collected at least two or more hours after the last meal in order to eliminate the effect of food and beverage on the sAA activity [21]. All subjects was instructed to refrain from brushing their teeth for at least 60 min before the measurement and and to rest in a chair for at least 10min [23]. The data collected was processed and analysed with SPSS©version 17. The level of significance was set at p<0.05.
2.3. Instruments
2.3.1. World Health Organization Alcohol, Smoking, and Substance Involvement Screening Test (WHO ASSIST) Version 3.0
- ASSIST was developed under the auspices of the WHO by an international group of addiction researches and clinicians to detected and managed drug use and related problems. The ASSIST obtains information from clients about lifetime use of substances, and use of substances and associated problems over the last 3 months. It can identify a range of problems associated with substance use including acute intoxication, regular use, dependent or ‘high risk’ use, and injection behavior.The ASSIST instrument contains the main points and instructions that guide the interviewer during interview. Currently ASSIST is only validated for use in an interview. The ASSIST (version 3.0) consists of 8 items questionnaire to screen the use of the following substances such as tobacco, alcohol, cannabis, cocaine, amphetamine type stimulant (including ecstasy, dexamphetamine, methamphetamine), inhalants, sedatives, hallucinogens, opioids, and ‘other’ dugs. Different scores could be summed for ASSIST such as specific substance involvement score and total substance involvement score. In the present study, we focused on specific substance involvement score for metamphetamin use by adding response score of question 2-7 for metamphetamine category. A risk score is provided for metamphetamine, and scores are grouped into low risk (0-3), moderate risk (4-26), or high risk (≥27) [1].
2.3.2. Device to Measure sAA
- Salivary AA was measured using a hand-held monitor (Nipro Co., Japan) that could measure the enzymatic sAA automatically using reagent paper. The hand-held monitor consisted of a disposable test-strip and a monitor designed by Yamaguchi et al [20, 21]. The monitor was equipped with a saliva transfer device and an optical device. The collecting paper was directly inserted into the oral cavity, and approximatelly 20-30 μl of whole saliva were collected from under the tongue in about 30s. Immediately after saliva collection, the test strip was placed onto the automatic saliva transfer device. The test-strip paper contained 2-chloro-4-nitrophenyl-4-o-β-D-galactopyranosylmaltoside (Gal-G2-CNP) and when Gal-G2-CNP is hydrolized by salivary amylase, the hydrolized product develops a yellow color over time by the following reaction. Using this hand-held monitor, it took 30 s for saliva sampling and 30 s for saliva transfer and measurement and so a total of 1 min was sufficient for measuring sAA activity [24].
3. Results
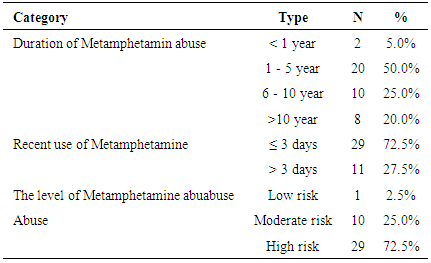
- Table 1 showed the characteristics of both metamphetamine abuser and healthy control groups. The majority of the subjects in metamphetamine abuser group were males (97.5%), more than half were married (65%) and worked (62.5%), and most subjects had formal education level until senior high school (60%). In control group, all subjects were males (100%), the majority had education level until senior high school (52.5%) and most were unmarried (77.5%) and unworked (55.0%).
|
|
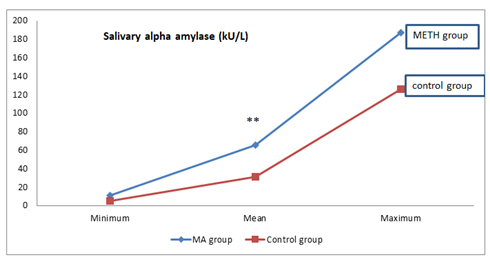 | Figure 1. Salivary AA level between METH group and control group (**p=0.001) |
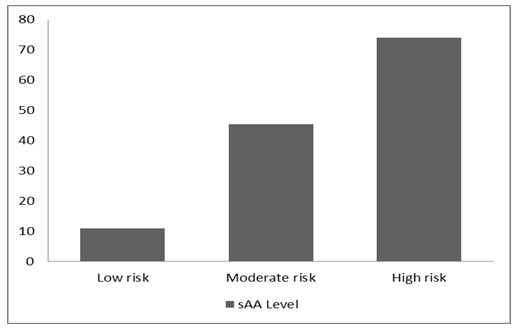 | Figure 2. Salivary AA levels in METH abusers |
4. Discussion
- From the subject characteristics, we found that majority of the metamphetamine users group were males and only one female. Similar results were obtained from a study conducted by Durell et al (2008) that methamphetamine abuse was more common in males (0.32%) than in females (0.23%) and according to UNODC (2013), that the majority methamphetamine users in Indonesia were male (89%) [3, 25]. Different results were obtained from a national survey of students in Brazil in 2009, amphetamine type stimulants (ATS) use were higher in female students (14.1%) than in men (5.5%), due to the effects of anorexia and weight loss derived from ATS as the purpose of female students [2].Epidemiologically substance abuse is higher in men (11.5%) than women (7.3%) [26]. Previous annual report of Rehabilitation Center of National Narcotic Board Baddoka in 2016, the majority substance abusers who undergo inpatient and outpatient rehabilitation were men [5]. Women were more likely obtained inadequate treatment for substance abuse than men. Low income, possible pregnancy, and child care responsibility make women experienced more barriers for seeking substance abuse rehabilitation. In addition, women are more likely hiding substance abuse due to social stigma and the risk of losing child [27].From occupational and marriage status, the percentage of methamphetamine abusers was found greater in married and worked. A previous report from Rehabilitation Center of National Narcotic Board Baddoka concluded that the largest proportion of drug abusers in Indonesia in 2011 around 3.7 to 4.7 million were worker and married [28]. Probably due to work and marriage could be a stressful condition and encouraged subjects to abuse substances.Methamphetamine potently activates the sympathetic nervous system by increasing central and peripheral norepinephrine. A number of studies in humans and animals have suggested that sympathetic activation caused by stress, exercise, pharmacological, or local neuronal stimulation, increases the release of sAA [15]. We found that the sAA levels in metamphetamine abusers were significantly higher than those of normal healthy controls. In this study, we also tried to compare the sAA levels among various levels of metamphetamine abuse. The highest mean levels of sAA was found on subjects who have high risk metamphetamine abuse level (73.93 ± 51.36 kU/l) followed by moderate risk (45.40 ± 30.67 kU/l) and low risk that only one subject (11.00 kU/ l). According to interpretation of ASSIST score, subject with low risk are at a lower risk of problems related to their substance use. While they may use substances occasionally, they are not currently experiencing any problems related to their use and are at lower risk of developing problems related to their substance use in the future with their current pattern of use. Subjects with moderate risk are at moderate risk of health and other problems, and indicates a likelihood of future problems, including the possibility of dependence. Subjects with high risk abuse are at high risk of dependence and are probably experiencing health, social, financial, legal and relationship problems as a result of their substance use [24]. Higher sAA levels were found in high risk subjects, because the noradrenergic response becomes sensitized or reactive due to the chronic activation of the locus coeruleus by chronic substance abuse (high risk of methamphetamine abuse). This result suggest that there was a chronic level of stress in high-level methamphetamine abusers. Previous study have reported an association of sAA activity with chronic stress [22].The authors were not able to compare this result with other study because based on the author's knowledge, there was no study comparing sAA levels at various levels of abuse. We found one published study conducted by Haile et al (2013) concluded that methamphetamine increased sAA activity in non treatment seeking METH-dependent volunteers who received administered intravenous METH (30mg) [19]. Although there was a difference in mean levels of sAA at various levels of abuse, but Spearman's correlation test showed no significant correlation between methamphetamine abuse level score (WHO ASSIST V3.0 score) and the sAA levels. This probably due to habituation of the SAM system in the use of chronic methamphetamine, leading to blunted of stress responses from the SAM system, but this remains to be investigated further. There was no study had examined the effects of chronic methamphetamine use on sAA activity, but there were several studies that examine the association of caffeine consumption (sympathetic nerve stimulants) to the sAA activity. They concluded there were no significant association between caffeine consumption and the sAA activity, and suggested the blunted responses to stress from SAM system on subjects consuming daily caffeine might be the reason [29-31].Interestingly, there was a significant correlation between the recent use of methamphetamine with the sAA level (r = -0.377, p<0.05). This result indicating that the methamphetamine levels in the body decreased due to the pharmacokinetic (excretory) process could influenced the sAA level. Further research is needed to examine wheter sAA measurement could be considered as a additional tool in detection of metamphetamine abuse. Previous study conducted by Haile et al (2013) showed that sAA activity was related to methamphetamine dose (p <0.001) [19]. In this study we also found that the duration of methamphetamine use was not correlated with sAA levels (r = 0.168, p> 0.05). There was no study examined the association duration of methamphetamine abuse with the sAA activity.The methodological limitation of this study was only one point measurement was used and we did not assessed the psychological stress experienced by methamphetamine abusers, which could also influenced the activity of sAA. Investigation of the association between psychological stress in metamphetamine abusers and sAA level is wararanted in future studies. The authors suggest further research with larger sample at each level of methamphetamine abuse to see the role of sAA enzyme levels as a tool for detecting methamphetamine abuse. Further research is needed also to see the correlation between levels of methamphetamine in plasma with salivary alpha amylase enzyme activity.
5. Conclusions
- The result of this study, there was no significant correlation between the level of methamphetamine abuse and the salivary alpha amylase levels. There were no difference of the sAA levels in low risk, moderate risk and high risk metamphetamine abusers. This might be due to the habituation of the SAM system in the chronic abuse of methamphetamine, leading to the blunted responses of the SAM system for stress, but needed a further investigation.We also found a significant correlation between the recent use of methamphetamine and the salivary alpha amylase enzyme levels. The closer to the measurement of the sAA, the higher sAA levels we got. Futher research was also needed for the role of salivary alpha amylase as an additional tool for detecting methamphetamine abuse.
ACKNOWLEDGEMENTS
- The authors declare no potential conflict of interest in writing this original article. The authors would like to acknowledge the important support and contributions of Uleng Bahrun M.D. (clinical pathologist) and R. Satriono, M.D. (Pediatrician; Clinical Nutrician; Research Methodology Advisor).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML