-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Clinical Psychiatry
2014; 2(1): 1-5
doi:10.5923/j.ijcp.20140201.01
The Association between Clinician’s Global Assessment of Functioning and Serum 25 (OH) Vitamin D Levels in Adult Veterans with Schizophrenia Residing in a Long-term Care Facility
Ian R. McGrane1, 2, Jennifer L. Zacher2
1Providence St. Joseph Medical Center Polson, MT, 59860, USA
2Captain James A. Lovell Federal Health Care Center, North Chicago IL, 60064, USA
Correspondence to: Ian R. McGrane, Providence St. Joseph Medical Center Polson, MT, 59860, USA.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Vitamin D is an important hormone that is well known for its role in musculoskeletal health. Data supports a role for vitamin D within the central nervous system as a neuroprotective and dopamine modulating steroid. It is hypothesized that vitamin D deficiency plays a role in the development of schizophrenia. There is increasing interest as to if serum vitamin D levels have an effect on disease state severity and functioning in patients with schizophrenia. The objective of this study was to identify an association between the physician’s global assessment of functioning (GAF) rating and serum 25(OH)D levels. A retrospective chart review was conducted using an electronic medical record. A total of 21 patients were included in this study. A negative association between serum 25(OH)D levels and GAF rating was found. Patients with serum 25(OH)D levels of ≤35 ng/mL were found to have an average GAF rating of 38 while patients with serum 25(OH)D levels of >35 ng/mL had an average GAF rating of 28 (p=0.041). No statistically significant difference between groups could be found when patients with diagnosis of dementia were removed from both groups. A total of 71% of all patients were determined to have sufficient vitamin D levels. Though it was found that patients with lower serum 25(OH)D levels had higher GAF ratings, a true inverse relationship cannot be accepted due to several limitations.
Keywords: Vitamin D, Schizophrenia, Long-term care, Veteran
Cite this paper: Ian R. McGrane, Jennifer L. Zacher, The Association between Clinician’s Global Assessment of Functioning and Serum 25 (OH) Vitamin D Levels in Adult Veterans with Schizophrenia Residing in a Long-term Care Facility, International Journal of Clinical Psychiatry, Vol. 2 No. 1, 2014, pp. 1-5. doi: 10.5923/j.ijcp.20140201.01.
Article Outline
1. Introduction
- Vitamin D is an important hormone that is best known for its role in musculoskeletal function. Measurable serum levels of vitamin D above a specific threshold have shown causality in the prevention of osteomalacia and rickets in humans [1]. Over 10 years of evidence has shown that vitamin D has additional roles in the body and suggests that it should be classified as a neuroactive steroid [2]. The role of vitamin D in the central nervous system (CNS) is still under extensive investigation, but current data suggests that 1,25(OH)2D3 and the vitamin D receptor (VDR) play a role in the developing brain [3]. This is believed to be done through alterations in neurotrophic signalling, an important function for the migration and development of brain cells. Additionally, brain morphology such as increased lateral ventricle size has been noted in vitamin D deficient mice [4]. The ventricle has high VDR density and is the primary site for brain neurogenesis [5]. Symptoms of behavioural abnormalities, such as novelty-induced locomotion, have been reported in vitamin D deficient animals [6-9] These symptoms are believed to be similar to positive symptoms of schizophrenia [3]. Additionally, increased novelty- induced locomotion is reliant upon subcortical dopamine function, where increase locomotion reflects hyperactive subcortical dopamine systems, as hypothesized in schizophrenia [10].Lower levels of vitamin D are associated with a multitude of medical and psychiatric diseases, including schizophrenia. Schizophrenia is a complicated disease thought to be related in part to CNS dopaminergic regulation. Risk factors contributing to low maternal vitamin D levels such as being born in the winter and spring months, living in urban environments, and having dark skin have shown robust association with the development of schizophrenia later in life [11-18]. Theoretical evidence to support a relationship between severity of disease and serum vitamin D levels is based on the dopaminergic modulating and neuroprotective properties vitamin D has shown [3,19]. Serum concentration of 25(OH) vitamin D is a useful clinical indicator of vitamin D metabolism, due to having a concentration half-life of roughly three weeks [20, 21]. However, there are inconsistencies worldwide as to the normal and optimal serum vitamin D range. The Institute of Medicine has recommended serum 25(OH)D levels less than 20 ng/mL to be considered deficient [22]. Vitamin D insufficiency has been defined as serum 25(OH)D levels between 21-29 ng/mL [23-29]. Serum 25(OH)D levels between 30 ng/mL and 100 ng/mL is considered sufficient [23]. The laboratory reference level for 25(OH)D at the Captain James A. Lovell Federal Health Care Center (FHCC) is consistent with the previous definitions. The ADVIA Centaur (Siemens Healthcare Diagnostics, Tarrytown, NY 2011) is the assay used for 25(OH)D levels at the FHCC. There is currently no established vitamin D level specific for patients with schizophrenia. This study seeks to identify an association between serum vitamin D levels and clinician’s global assessment of functioning within an adult veteran population with schizophrenia residing in a long-term care facility.
1.1. Study Objectives
- The objective of this study was to identify an association between the physician’s global assessment of functioning (GAF) score between patients with serum 25(OH)D levels in two different concentration ranges. Additionally, it is important to address serum 25(OH)D sufficiency status, which is reported descriptively.
2. Materials and Methods
- This study was a retrospective chart review with two patient groups: those with serum 25(OH) vitamin D levels greater than 35 ng/mL or and those with levels 35 ng/mL and less. An electronic chart review was conducted using the Computerized Patient Records Systems (CPRS) to obtain demographics, clinical, and pharmacy data.
2.1. Criteria for Inclusion
- Patients included were a) veterans at the FHCC who were 18 years of age or older at date of admission with a diagnosis of schizophrenia b) admitted to the FHCC Community Living Center (CLC) c) had at least one serum 25(OH)D level and GAF rating within 360 days of each other recorded in the FHCC electronic medical record. If the patient had more than one set of data meeting inclusion, only the earliest inclusive data for that year (beginning with January 1st and ending in December 31st) were included; this was determined by the GAF date. If more than one 25(OH)D levels was available for a single GAF rating, the vitamin D level in closest proximity to the GAF date was used. Patients excluded from the study had current diagnosis of schizoaffective disorder or other affective disorder and patients who were non-veterans.
2.2. Statistical Analysis
- The relationship of vitamin D level and GAF score was assessed using a linear relationship model and two-tailed student’s t-test. A total of 55 patients in each study arm were determined to be the minimum number of patients needed to achieve 95% confidence. Descriptive statistics were used for patient characteristics and percentage of patient serum 25(OH)D adequacy.
3. Results
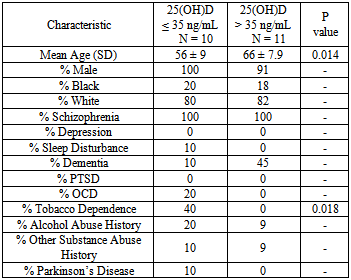
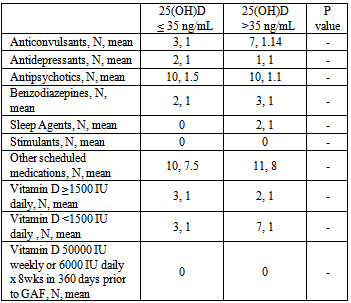
- A total of 21 patients met criteria for inclusion. Patient characteristics and pathology can be found in Table 1. The majority of patients were white males with schizophrenia. Patient characteristics were similar between each group, with the exception that patients were more aged in the >35 ng/mL 25(OH)D group than in the ≤ 35 ng/mL 25(OH)D group. Mental health pathology was similar between both groups, with the exception that there were a greater percentage of patients with dementia in the >35 ng/mL serum 25(OH)D group (p = 0.078). Patient medication information can be found in Table 2. The number of scheduled psychotropics, non-psychotropics, and vitamin D supplementation was similar between both groups. Patients with serum 25(OH)D levels of ≤ 35 ng/mL were found to have an average GAF rating of 38 while patients with serum 25(OH)D levels of >35 ng/mL had an average GAF rating of 28. This difference was found to be statistically significant (p=0.041). When patients with diagnosis of dementia were removed from both groups, no statistically significant difference could be found using multiple 25(OH)D cut-off points (data not shown). It was found that one patient had vitamin D deficiency, five patients had insufficiency, and 15 had adequate levels. All six patients who were determined to have deficient or insufficient vitamin D status were not receiving vitamin D treatment dosing of at time of GAF rating.
|
|
4. Discussion
- An inverse relationship was found between the clinicians GAF rating and serum 25(OH)D level in 21 adult veterans with schizophrenia residing in a community living center. Patients with serum 25(OH)D levels >35 ng/mL had an average GAF rating of 28 while patients with levels ≤ 35 ng/mL had an average GAF rating of 38. This finding was statistically significant (p=0.041) and is considered to be clinically significant as a GAF rating of 21-30 indicates a) behavior considerably influenced by delusions or hallucinations or b) serious impairment in communication or judgment or c) inability to function in almost all areas; while GAF ratings between 31-40 indicate a) some impairment in reality testing or communication or b) major impairment in several areas, such as work or school, family relations, judgment, thinking, or mood [30].Patients included in the study had numerous psychiatric diagnoses and were similar in many respects between the two serum 25(OH)D concentration arms. Though patient characteristics and pathology were found to be similar in most instances, the diagnosis of dementia was made in 45% of patients in the high vitamin D group versus in 10% of patients in the low vitamin D group (p=0.078). While this difference was not found to be statistically significant, dementia can be a highly debilitating disease, as criterion that cognitive decline must be present in order to meet diagnostic criteria [30]. An assessment of performance of everyday actions using the Naturalistic Action Test was conducted comparing patients with schizophrenia, dementia, and normal controls. It was found that both patients with schizophrenia as well as dementia exhibited impaired performance compared to normal controls, and that impairments in patients with schizophrenia and dementia were due to different mechanisms [31]. This data could support that patients in our study with diagnoses of both schizophrenia and dementia could exhibit greater cognitive dysfunction than schizophrenia alone. When patients with a diagnosis of dementia were removed from both vitamin D arms, no difference in GAF rating could be found between 25(OH)D groups using multiple cut-off points. Additionally, the average age of patients in the >35 ng/mL vitamin D group was 10 years greater than patients in the ≤ 35 ng/mL vitamin D group (p = 0.014). Increased age could indicate more severe disease states as well as worsening cognition. In order to control for differences in GAF scores due to age, patients were assessed and divided by age cut-offs of 60, 65 and 70 years. No statistically significant difference in GAF scores were found at any of these age cut-off points (p >0.05).Few studies have evaluated disease severity in association with serum vitamin D levels in patients with schizophrenia or psychosis. Gracious and colleagues assessed the relationship between 25(OH)D levels and severity of illness defined by the presence of psychotic features in 104 mentally ill adolescents at time of acute hospitalization admission. Psychotic adolescents were found to have a lower serum 25(OH)D level than non-psychotic adolescents (20.4 ng/mL vs. 24.7 ng/mL; p=0.04). Children with low vitamin D levels had an odds ratio of 3.5 (CI 1.4-8.9; p <0.009) of also showing psychotic features [32]. An additional trial compared serum 25(OH)D levels between three patient cohorts: adult patients with schizophrenia (n=50), depression (n=33) and healthy controls (n=50). Lower serum vitamin D levels were found in patients with schizophrenia compared with patients with depression and healthy controls. The Positive and Negative Syndrome Scale (PANSS) was administered to patients with schizophrenia, with no statistically significant differences found between PANSS score and vitamin D level [33]. The findings of both studies were unable to identify causality between lower serum 24(OH)D levels and increased severity of mental illness. McGrath and colleagues examined the association between neonatal 25(OH)D levels and schizophrenia status later in life using dried neonatal blood samples of 424 individuals with schizophrenia and 424 matched controls. The authors identified a non-linear relationship where persons in the lowest vitamin D quintiles (<40.5 ng/mL) had a 2-fold increased risk of schizophrenia. Additionally, it was found that the highest vitamin D quintile (>51 ng/mL) had an increased risk of schizophrenia. The authors speculate that a subgroup of the population may have a single-nucleotide polymorphism (SNP) which could influence the conversion of 25(OH)D3 to the active form of 1,25(OH)2D3, which may lead to increased risk of prenatal infection [34].Our findings are limited by numerous cofounders. The study was limited to a mostly white, male, veteran population and results cannot be generalized across a broad population. These findings are significantly limited by the small sample size and must be confirmed in a larger patient population. The use of the GAF score limits the specificity of clinical outcomes measured in schizophrenia. The GAF rating scale has been criticized for its lack of specificity and subjectivity of scoring and has been removed from inclusion in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-V) [35, 36]. The GAF ratings used in this study were provided by medical students, psychiatry residents, and attending psychiatrists. While all documented GAF scores in CPRS was viewed and co-signed by the attending psychiatrist, inexperienced practitioners could have led to inconsistencies in GAF scoring. The use of a more specific tool to schizophrenia such as the Positive and Negative Syndrome Scale (PANSS) would likely be able to better measure a larger spectrum of outcomes in patients with schizophrenia. Additionally, misclassification of serum 25(OH)D level could have occurred as slight variability has been reported in the literature. The limit of quantitation (LOQ), or concentration at which quantitative results can be reported with a high degree of confidence is 4.2 ng/mL for the ADVIA Centaur assay used at the FHCC [37]. However, it was found that misclassification of vitamin D status occurred in roughly 20-33% of serum samples using another common laboratory assay, the DiaSorin Liaison method, compared with the gold standard assay, Liquid Chromatography Tandem-Mass Spectrometry (LC-MS/MS). However, no direct assay comparison could be found between the ADVIA Centaur assay and LC-MS/MS [38]. Finally, co-morbidity ratings were not accounted for in this study.
5. Conclusions
- Though it was found that patients with lower serum 25(OH)D levels had higher GAF ratings, a true inverse relationship cannot be accepted at this time due to a small sample size and a number of confounding factors. This study contributes to a growing number of negative trials for vitamin D status having a strong associative benefit in schizophrenia. A large study evaluating sustained vitamin D levels over many years with clinical evaluations using both specific and general assessment tools for treatment naïve patients would be needed to assess the role of vitamin D’s clinical effects in schizophrenia. However, it was found that of the 29% of patients without sufficient vitamin D status, treatment dosing was not conducted at the time of GAF rating. This suggests that even though vitamin D status may not play a role in global functioning, these patients may not be receiving adequate supplementation for bone health maintenance.
Conflict of Interest Statement
- The authors have no conflicts of interest to disclose, regarding, but not limited to: place of employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications, grants, or other funding.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML