-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biological Engineering
p-ISSN: 2163-1875 e-ISSN: 2163-1883
2016; 6(1): 1-6
doi:10.5923/j.ijbe.20160601.01

Growth Characteristics of Newly Isolated Xylose-Assimilating Bacterium and Accumulation of Green Plastic, Polyhydroxyalkanoate in the Genetic Engineered Strain
Kenji Tanaka1, Shunya Mori1, Kastuki Matsumoto1, Kojiro Yamamoto1, Izumi Wakida2, Saki Goto2, Hiromi Mastusaki2
1Department of Biological and Environmental Chemistry, Faculty of Humanity-Oriented Science and Engineering, Kindai University, Kayanomori, Iizuka, Japan
2Department of Food and Health Sciences, Faculty of Environmental and Symbiotic Sciences, Prefectural University of Kumamoto, Tsukide, Kumamoto, Japan
Correspondence to: Kenji Tanaka, Department of Biological and Environmental Chemistry, Faculty of Humanity-Oriented Science and Engineering, Kindai University, Kayanomori, Iizuka, Japan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A bacterial polyester, polyhydroxyalkanoate (PHA) is expected as “green plastic” because it is thermoplastic, flexible and biodegradable. In commercial production of PHA, the use of economic and ecological substrate like biomass is necessary. Hemicellulose, which is mainly composed of xylose and other monosaccharides can be a candidate of substrate for production of PHA. However, xylose is not easily utilized by microorganisms. We isolated a bacterium that grows at a high specific growth rate in xylose-mineral salts medium. The bacterium, which was named Enterobacter sp.TF, assimilates many kinds of sugar but it does not accumulate PHA. Hence, we made the genetic recombinants by introducing the genes for biosynthesis of homopolyester of D-3-hydroxybutyrate, PHB from Ralstonia eutropha, and the genes for biosynthesis of PHA copolyester from Pseudomonas sp.61-3. As a result, the recombinant introduced with the genes of R.eutropha accumulated PHB from xylose.
Keywords: Enterobacter, Green plastic, PHB, Xylose
Cite this paper: Kenji Tanaka, Shunya Mori, Kastuki Matsumoto, Kojiro Yamamoto, Izumi Wakida, Saki Goto, Hiromi Mastusaki, Growth Characteristics of Newly Isolated Xylose-Assimilating Bacterium and Accumulation of Green Plastic, Polyhydroxyalkanoate in the Genetic Engineered Strain, International Journal of Biological Engineering, Vol. 6 No. 1, 2016, pp. 1-6. doi: 10.5923/j.ijbe.20160601.01.
1. Background
- Xylose is one of main component sugars of hemicellulose. For utilization of lignocellulosic biomass, it is important to convert xylose to useful compounds efficiently and cost-effectively. However, xylose is not easily utilized by microorganisms and the variety of fermentation products from xylose is small. It is expected to enlarge the variety of fermentation products by using newly isolated or engineered microorganisms. Poly(D-3-hydroxyalkanoate) (PHA) is the polyester accumulated in bacterial cells under imbalanced nutritional condition like nitrogen limitation. PHAs are expected as raw material for manufacturing of green plastic because they are thermoplastic, flexible and biodegradable. Poly(D-3-hydroxybutyrate) (PHB) was the first reported and well-known PHA. However, PHB is inferior in flexibility, then it is difficult to mold and fragile to stress of physical force. Therefore, the copolyester which is composed of both D-3-hydroxybutyrate and other types of hydroxyalkanoates are expected. In most of the researches for production of copolyester PHA, fatty acid, plant oil and hexose like glucose or fructose have been used as the substrate [1-11]. In this study, we investigated the fermentation characteristics of our newly isolated xylose-utilizing bacterium and the production of polyester from xylose in the genetically engineered strain.
2. Methods
- Isolation and identitication of xylose-assimilating bacteriumThe water sampled from Onga River, which is flowing in the north area of Kyushu island, Japan, was spread on the mineral salts agar plate containing xylose as the sole carbon source and then it was incubated for 7 days at 30°C. Xylose-mineral salts agar medium was composed of (NH4)2SO4 2.0g, MgSO4・7H2O 0.2g, KH2PO4 1.0g, xylose 20g, agar 15g and trace elements solution 0.1mL per 1 L of distilled water. The composition of the trace elements solution was FeSO4・7H2O 16.0g, CrCl3・6H2O 0.13g, NiCl3・6H2O 0.18g, sodium citrate dihydrate 15.6g, CuSO4・5H2O 0.1g, CoCl2・6H2O 15.6g in 100mL of 1M HCl. pH of the culture medium was adjusted to 6.8. After several trials, one bacterial colony was obtained. Morphological and physiological tests were carried out for identification of the isolated bacterium. Homology for nucleotide sequence of the 16S rRNA gene against other bacteria was also investigated according to the scheme that we reported previously [12]. Culture testGrowth characteristics of wild strain and the genetically engineered strains of our isolated bacterium were investigated by flask culture and pH-controlled batch culture. pH-controlled batch culture experiment was carried out using a glass jar fermenter (total volume 1000mL; working volume 600mL) equipped with a pH controller (PHC-2201, Biott Co., Ltd., Tokyo) connected to a tube pump for feeding 4% ammonia water. Aseptic air was supplied through a sterile filter (i.d 0.2μm) at a flow rate of 0.5vvm to the fermenter during cultivation. The agitation speed was kept at 1200rpm. As the fermentation proceeded, the vigorous foaming of culture liquid occurred in the fermenter. Hence, a defoaming agent (Einol, Biott Co. Ltd., Tokyo) was periodically added to prevent the culture liquid overflowing from the fermenter.Cell growth was monitored by measuring the optical density at 600nm (OD600) of the culture liquid. The polyester accumulated in the cells was determined according to the method using gas chromatography [13]. The lyophilized cells, a mixture of 2mL of methanol acidified with 3% (w/v) H2SO4 and 2mL of chloroform were added into a screw cap vial and then it was heated at 100°C for 3.5h for degradation of the polyester, and esterification of hydroxyalkanoic acid and methanol. After cooling, 1mL of H2O was added into a vial then the suspension was shaken well for 10min. After two phases were allowed to separate, the organic phase containing the methyl ester was applied to gas chromatography.DNA manipulation and plasmid constructionIn this study, three types of recombinant plasmids were introduced into Enterobacter sp.TF, respectively:- (i) The plasmid pRKmKSc-C1GAB was constructed to introduce the following genes for biosynthesis of PHA: β-ketothiorase gene (phbA) and acetoacetyl-CoA reductase gene (phbB) from R.eutropha to provide D-3-hydroxybutyrate, D-3-hydroxyacyl-acyl carrier protein (ACP)-CoA transferase gene (phaGPs) from Pseudomonas sp. 61-3 to provide various D-3-hydroxyalkanoates, PHA synthase gene (phaC1Ps) having broad substrate specificities from Pseudomonas sp. 61-3 to polymerize various D-3-hydroxyalkanoates, and lac promotor. (ii) The plasmid pRKmKSc-C1G was constructed by eliminating phbA and phbB from the plasmid pRKmKSc-C1GAB. (iii) The plasmid pRKmBB-phbCAB, which is containing the genes for biosynthesis of PHB from R.eutropha (phbCABRe), was also used. All the plasmids used in this study are shown in Table 1.
|
3. Results
- Identification of the isolated bacterium and assimilation of carbon sourceThe bacterium, which was isolated from Onga River with xylose-mineral salts agar plate, was Gram negative, straight rod (1.0–1.5μm), motile and facultatively anaerobic. Hydrolysis of gelatin and urease was also observed. A scanning electron microscope image of the isolated bacterium is shown in Fig.1.
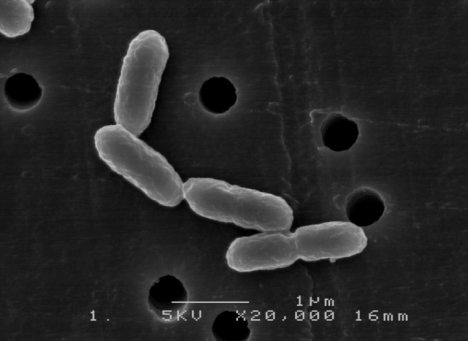 | Figure 1. SEM image of isolated xylose assimilating bacterium |
|
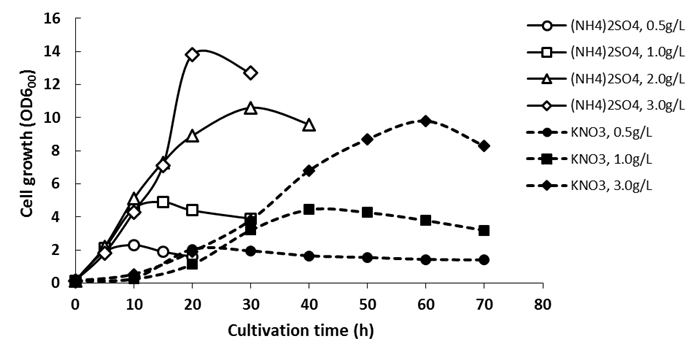 | Figure 2. Cell growth of Enterobacter sp. TF in flask culture using (NH4)2SO4 or KNO3 with different concentrations as nitrogen source. Initial xylose concentration was  |
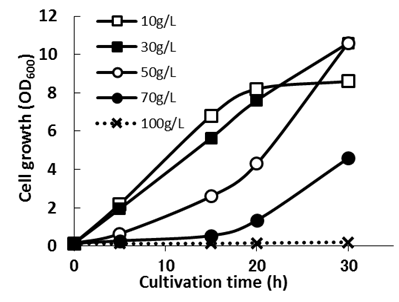 | Figure 3. Cell growth of Enterobacter sp. TF in flask culture using culture medium with different xylose concentraions |
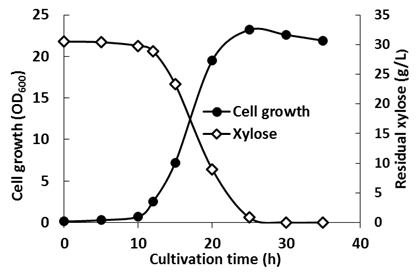 | Figure 4. Fermentation time course of Enterobacter sp. in pH-cntrolled batch culture with feeding 4% ammonia water. pH was maintained at 6.3 |
|
4. Discussion
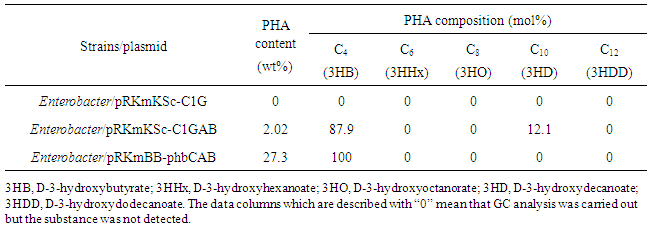
- The enzyme PhaG is a D-3-hydroxyacyl-acyl (3HA) carrier protein (ACP)-CoA transferase providing various D-3-hydroxyacyl-CoAs from fatty-acid biosynthetic pathway as the substrate of PHA. However, the accumulation of PHA or PHB in the two recombinant strains incorporated with the gene phaG was very poor. Enterobacter/pRKmKSc-C1G did not accumulate polyester in the cells while Enterobacter/pRKmKSc-C1GAB accumulated slight amount of poly(D-3-hydroxybutyrate-co-D-3-hydroxydecanoate). On the other hand, the Enterobacter/pRKmBB-phbCAB, which has no phaG, accumulated PHB in the cells over 20wt%. Recently, Wang et al. reported that the enzyme PhaG is not only functioning as 3HA carrier protein (ACP)-CoA transferase but also functioning as 3-hydroxyacyl-ACP thioesterase [5]. Therefore, in the two recombinants incorporated with phaG, D-3HA-CoA formed in the cells would be immediately converted to free D-3-hydroxyalkanoic acids, which caused the decrease in intracellular content of polyester. Actually, HPLC analysis for culture supernatant of the Enterobacter/ pRKmKSc-C1GAB indicated that several kinds of organic acids like pyruvate and L-malate are excreted from the cells and the amount is not small (the data is not shown). It is known that xylose is metabolized mainly via pentose phosphate (PP) pathway/glycolytic pathway or phosphoketolase (PK) pathway. In case of some lactic acid bacteria, the PK pathway yielding acetate, formate and ethanol, and the PP/glycolytic pathway converts xylose to L-lactate only. Furthermore, it is also reported that the change in the xylose concentration shifts the metabolism between the PK pathway and the PP/glycolytic pathway and pyruvate metabolism between cleavage to acetyl-CoA and formic acid by pyruvate-formate lyase and the reduction to L-lactate by lactate dehydrogenase, therefore the yield coefficient of lactate/xylose changes according the xylose concentration [22]. In recent years, many researchers have reported for microbial production of PHB and copolyester PHA from xylose or other pentose derived from hemicellulose, for instance, engineered E.coli [23] and yeast [24], mcl-PHA production from xylose and octanoic acid by engineered Pseudomonas putida KT2440 [25], screening of bacteria to produce PHB from xylose [26], PHA production from sugar maple hemicellulosic hydrolysate by Burkholderia cepacia [27], and PHA production from lignocellulosic materials [28]. In those reports, the yield, content and productivity of PHB or PHAs from xylose were still lower compared to those from glucose or organic acids.To increase the content of PHA in the engineered Enterobacter sp.TF and the molar ratio of second unit, it is necessary to elucidate the metabolic pathway of xylose. It is also expected that the introduction of D-3-hydroxyacyl (3HA)-CoA ligase gene, which is available to provide mcl-3HA units efficiently to polymerization from fatty acid biosynthesis pathway, may improve the accumulation of copolyester.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML